Abstract
CsPbBr3:xBi3+ quantum dot glass was prepared by using traditional melting-quenching and heat treatment processes. The effects of Bi doing on the sinter of the precursor of glass and the crystalline of the perovskite quantum dot were discussed detailly. By doping Bi2O3 into the borosilicate glass matrix, the melting temperature was reduced to 900 °C. The tunable emission of CsPbBr3 quantum dots from 523 to 493 nm was achieved with suitable Bi2O3 doping. Due to the protection of the inorganic glass matrix, the prepared CsPbBr3 quantum dots still exhibit excellent thermal stability after multiple thermal cycles and thermal shocks. This provides a good solution to the problem of poor thermal stability of perovskite quantum dots.
Export citation and abstract BibTeX RIS
The application of cesium halide lead perovskite (CsPbX3, X = Cl, Br, I) quantum dots (QDs) in luminescence has achieved unprecedented rapid progress in electronic devices such as light-emitting diodes (LEDs), photodetectors, visible light communication, and solar cells, 1–6 due to its tunable band gap, large defect tolerance, the resulting high efficiency and narrow band photoluminescence cover the entire visible range. 7–16 Nevertheless, due to the ionic nature and large surface energy of CsPbX3 QDs, its long-term stability has been seriously affected. 17
A lot of strategies have been created to solve above problem. At present, embedding the QDs into glass is considered to be a very effective method for guarantying the excellent optical performance and long-term stability of QDs. 18–25 The most commonly used inorganic glass is silicate glass, whose structure is a continuous silica tetrahedron. The stable Si–O tetrahedron structure contributes to the stability of the glass and provides a stable environment for QDs. 26,27 As we all know, the temperature required to prepare borosilicate glass must reach at least 1000 ℃, but PbX2 has a boiling point between 900 ℃–1000 ℃, one raw material of CsPbX3 QDs. Therefore, it is necessary to decrease the melting point of the borosilicate glass. The melting point of Bi2O3 is only 825 ℃, we propose that on the premise of preparing QDs with silicate glass as the protective matrix, doping with Bi2O3 can lower the melting point and thus reduce the volatilization of PbX2. Otherwise, impurity doping is widely used to impart new optical, electronic, magnetic and thermal functions to semiconductor materials. 28–30 Chen et al. introduced fluorine into oxyhalide borosilicate glass by doping, breaking the tight network of the glass and promoting the growth of CsPbX3 (X = Cl, Cl/Br, Br, Br/I, I) in the glass. 31 In this work, we successfully prepared CsPbBr3: xBi2O3 QDs glass and discussed the optical performance detailly.
Experimental
Sample preparation
Using sodium carbonate anhydrous (Na2CO3, ≥99.5%), barium carbonate (BaCO3, 99%), silicon dioxide (SiO2, 99.5%), bismuth trioxide (Bi2O3, ≥99%), zinc oxide (ZnO, 99%), boracic acid (H3BO3, ≥99.5%), caesium carbonate (Cs2CO3, 99.99%), lead oxide (PbO, ≥99%), and sodium bromide (NaBr, 99%) as the starting material, prepare CsPbBr3:xBi3+ perovskite QDs, where x is the molar ratio of Bi2O3. The QDs were precipitated in borosilicate glasses using the similar process as reported previously, 7 while the melting process was controlled as 900 °C in the atmosphere for 15 min and the heat-treatment with a temperature of 430 °C for 3 h. Finally, the obtained CsPbBr3: xBi2O3 QDs glass was optically polished for further characterization and use.
Results and Discussion
In this work, 5Na2CO3-20ZnO-80H3BO3-15SiO2-10BaCO3-XBi2O3 (X = 0, 6, 12, 24, 48, 72 mol%) borosilicate glass composition with 1.5Cs2CO3-3PbO-9NaBr(mol%) perovskite-related sources was used as the raw materials to prepare QDs glass. The X-ray diffraction (XRD) patterns of perovskite quantum dot glasses doped with different Bi2O3 molar ratios are shown in Fig. 1a. All diffraction peaks can correspond to the standard card CsPbBr3 (PDF#54-0752) without any obvious secondary phase. Simultaneously, the diffraction peak did not change significantly with Bi2O3 doping. As shown in Fig. 1b, the CsPbBr3 crystal has a cubic structure with a space group of Pm
 m. CsPbBr3 QDs are successfully embedded in the glass matrix as depicted in Fig. 1c. High resolution TEM (HRTEM) micrograph (Fig. 1d) clearly shows the crystallinity and lattice fringes of CsPbBr3 particles. The interplanar spacing is 2.38 Å, which is consistent with the (211) plane of cubic CsPbBr3. The results indicate that the CsPbBr3 QDs were successfully grown in the borosilicate glass matrix, and the doped Bi3+ ions did not enter the CsPbBr3 nanocrystals.
m. CsPbBr3 QDs are successfully embedded in the glass matrix as depicted in Fig. 1c. High resolution TEM (HRTEM) micrograph (Fig. 1d) clearly shows the crystallinity and lattice fringes of CsPbBr3 particles. The interplanar spacing is 2.38 Å, which is consistent with the (211) plane of cubic CsPbBr3. The results indicate that the CsPbBr3 QDs were successfully grown in the borosilicate glass matrix, and the doped Bi3+ ions did not enter the CsPbBr3 nanocrystals.
Figure 1. (a) The XRD spectra of the obtained glass under different Bi2O3 doping concentrations and the standard spectra of CsPbBr3 (PDF-540752). (b) Cubic crystal structure of CsPbBr3. (c) TEM image of the CsPbBr3: xBi3+(x = 0.06) sample. (d) HRTEM image of Bi3+ doped CsPbBr3 quantum dot glass and corresponding crystal size distribution, the d is mean the distance of the (211) crystal face of CsPbBr3 quantum dot.
Download figure:
Standard image High-resolution imageThe variation of the normalized PL spectra of CsPbBr3: xBi2O3 (x = 0, 0.06, 0.12, 0.24, 0.48, 0.72) excited by 365 nm light are shown in Fig. 2a. Without doped with Bi2O3, the CsPbBr3 perovskite quantum dot glass sample at this time has a maximum emission peak at 523 nm. With the gradual increase of the content of doped Bi2O3, the emission peak gradually decreased. When the doping concentration x = 0.48, the emission peak reached the lowest value, and the emission intensity was almost negligible. In addition, by normalizing the emission intensity, it was found that with the increase of the doping concentration of Bi2O3, the emission peak has a blue shift from 523 nm to 493 nm. The reason for this phenomenon is that with the addition and increase of Bi3+ ions, the matrix of the glass network structure does not change, and Bi3+ ions just enter the vacancies of the glass network structure, which will reduce the growth of CsPbBr3 quantum dot occupying vacancies Probability, which in turn leads to a decrease in emission intensity. At the same time, when Bi3+ ions and CsPbBr3 quantum dot coexist in the same vacancy, the growth space of CsPbBr3 quantum dot becomes smaller due to the existence of Bi3+ ions, resulting in a smaller size of CsPbBr3 quantum dot, resulting in a blue shift of the emission peak.
Figure 2. (a) The normalized PL spectra of the samples at different molar ratios of Bi2O3 excited by 365 nm UV light. (b) The absorption spectra of the samples at different molar ratios of Bi2O3. (c) Photographs (top: under daylight, bottom: under UV irradiation).
Download figure:
Standard image High-resolution imageIn further experiments, we measured the UV–vis absorption spectrum of CsPbBr3 quantum dot glass samples. As shown in Fig. 2b, it can be seen that without doping Bi2O3, the absorption curve of the CsPbBr3 quantum dot sample has a strong absorption at 523 nm, which corresponds to its emission spectrum, which the direct exciton recombination luminescence of CsPbBr3 quantum dot in the glass. When a small amount of Bi2O3 (x = 0.06) is doped, the absorption curve of the CsPbBr3 quantum dot sample at this time will have a slight blue shift, and the absorption intensity will also decrease accordingly. This is consistent with the performance of the emission spectrum, which is all due to the doping of Bi3+ ions into the vacancies of the grid structure of the glass matrix, which leads to the reduction of the size and number of CsPbBr3 quantum dot and the increase of the band gap. When a certain amount of Bi2O3 (x = 0.12, 0.24) is doped, the absorption curve has an obvious blue shift, and the absorption peak intensity is around 480 nm. Through searching for information, we found that the doped Bi3+ ion will produce an emission peak at 480 nm. Therefore, the absorption peak produced by the absorption curve at 480 nm is mainly derived from Bi3+ ions in the glass matrix. When excessively doped Bi2O3 (x = 0.48, 0.72), the absorption curve shows a large-span red shift, which is due to the self-absorption phenomenon of the Bi3+ ion in the glass matrix to the CsPbBr3 quantum dot. This is the reason why CsPbBr3:xBi3+(x = 0.48, 0.72) samples hardly emit light under 365 nm excitation. Figure 2c shows the photo of the CsPbBr3: xBi3+ glass sample under daylight and UV irradiation. When the doping concentration of Bi2O3 is 0.72 mmol, the prepared CsPbBr3: xBi3+ sample hardly emits light, which corresponds to the result of the emission spectrum.
In addition, in order to more directly understand the influence of Bi2O3 on the luminescence of CsPbBr3, we measured the photoluminescence quantum yield (PLQY). As shown in Fig. 3a, when the sample is not doped, the quantum yield is 60.8%. As the doping concentration of Bi2O3 gradually increases, the quantum efficiency of the sample gradually decreases. When the doping concentration of Bi2O3 reaches 0.72 mmol, the quantum efficiency is only 0.8%, the PLQY result is consistent with the emission spectrum. What is more, we monitored the time-resolved PL decay curve of Bi2O3 quantum dot glass samples doped with different concentrations as depicted in Fig. 3b. Using the double exponential fitting function to fit the attenuation curve well is as follows:
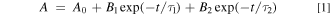
where A is phosphorescence intensity at time t, and B1/B2 and τ1/τ2 are the weight coefficient and decay time, respectively. And the fitting formula of average life is:
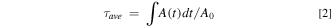
In the formula, A0 is the peak intensity, and A(t) is the PL intensity related to the recording time.
Figure 3. (a) PLQY of CsPbBr3 quantum dot glass with different concentrations of Bi2O3. (b) The decay curve of CsPbBr3 quantum dot glass with different concentrations of Bi2O3.
Download figure:
Standard image High-resolution imageAccording to previous studies, 32 the double exponential fitting results of the fluorescence lifetime of perovskite nanocrystals can be divided into fast decay time and slow decay time. The slow decay time may be related to the radiative recombination used for luminescence, and the fast decay time part may be related to the non-radiative recombination when the surface electrons are captured by the defect state and lost in the non-radiative process. As shown in Table I, we can observe that the value of the slow decay time decreased from 9.943 ns (56.66%) for the undoped sample to 2.548 ns (18.37%) for the 0.72 mmol sample. At the same time, as the Bi2O3 doping concentration gradually increases, the average life of the sample gradually decreases, which is consistent with the results of luminescence intensity and quantum yield.
Table I. The fluorescence lifetime of the sample after double exponential fitting and the average lifetime under different Bi2O3 concentrations.
| Doped (mmol) |
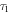 (ns) (ns) |
 (ns) (ns) |
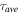 (ns) (ns) |
|---|---|---|---|
| 0 | 1.2640 (43.34%) | 9.943 (56.66%) | 6.18152140 |
| 0.06 | 0.9038 (42.65%) | 6.223 (57.35%) | 3.95436120 |
| 0.12 | 0.5852 (36.93%) | 5.346 (63.07%) | 3.58783656 |
| 0.24 | 0.2580 (70.61%) | 2.351 (29.39%) | 0.87373270 |
| 0.48 | 0.2990 (80.60%) | 2.666 (19.40%) | 0.75819800 |
| 0.72 | 0.2776 (81.63%) | 2.548 (18.37%) | 0.69467248 |
The XRD test has confirmed that the doped Bi3+ does not enter the CsPbBr3 quantum dot lattice. In order to verify that the doped Bi3+ ions only enter the vacancies of the glass network structure, we measure the infrared spectrum of the glass sample to determine the influence of Bi3+on the composition and structure of the glass matrix. As shown in Fig. 4, it shows the FT-IR spectra of a glass sample not doped with Bi2O3 and a glass sample doped with 0.24 mmol Bi2O3 at 400–4000 cm−1 wavenumber. And it can clearly show the vibration types and wave numbers of [BO3], [BO4] and [SiO4] units in the glass matrix. The absorption peak near 510 cm−1 is attribute to the bending vibration of the B–O–B bond. The absorption peak near 730 cm−1 is comes from the B–O–B bond bending vibration in the [BO3] triangle. The absorption peak at 1000 cm−1 belongs to the stretching vibration of [SiO4] tetrahedron. The absorption peak near 1240 cm−1 is mainly ascribed to the vibration of the boron oxygen ring. And the absorption peak near 1375 cm−1 is mainly derived from the asymmetric stretching vibration of the B–O–B bonds in the [BO3] triangle. Comparing the infrared spectra of a glass sample not doped with Bi2O3 and a glass sample doped with 0.24 mmol Bi2O3, it can be found that the vibration type and wavenumber of the glass matrix [BO3], [BO4] and [SiO4] groups have almost no change. Therefore, we believe that the doping of Bi2O3 does not change the composition and structure of the glass matrix. Motivated by this, it is indirectly confirmed that the doped Bi3+ ions only enter the vacancies of the glass network structure.
Figure 4. IR spectra of a glass sample not doped with Bi2O3 and a glass sample doped with 0.24 mmol Bi2O3.
Download figure:
Standard image High-resolution imageThe stability of the prepared CsPbX3 quantum dot, including light stability, thermal stability and water resistance stability, is the main factor restricting its application. 33–35 In 2019, Shao et al. have confirmed that using borosilicate as a glass matrix is the most effective measure to improve the stability of borosilicate nanocrystals in water or other environments, and it is also the best way to protect high borosilicate nanocrystals. 36 In order to determine the potential applications of the prepared CsPbBr3 quantum dot glass in lighting and display, the stability and reliability of the CsPbBr3:xBi3+ glass samples were studied. By heating/cooling the glass sample of CsPbBr3:xBi3+ (x = 0.06), the temperature is increased from room temperature 25 ℃ to 125 ℃, and the measurement is performed every 10 ℃. The PL intensity of the sample changes with temperature is recorded to study the sample's thermal stability, as depicted in Fig. 5a. From the temperature and emission intensity, it can be found that after a heating/cooling cycle, the emission intensity hardly changes. In order to further study the reliability of CsPbBr3:xBi3+ samples, under the same conditions, we conducted 10 heating and cooling shock tests on CsPbBr3:xBi3+ (x = 0.06) samples. As shown in Fig. 5b, the emission peak intensity after 10 consecutive cycles remains basically unchanged. The results of heating and cooling cycling and heating and cooling shock show that the prepared CsPbBr3:xBi3+ sample is very stable, without obvious degradation and size changes.
Figure 5. (a) The emission intensity of the CsPbBr3:xBi3+ (x = 0.06) sample with temperature changes is obtained through a heating/cooling cycle of 25 to 125 ℃. (b) The emission intensity of the CsPbBr3:xBi3+ (x = 0.06) sample at 25 ℃–125 ℃ for 10 times of heating and cooling shock.
Download figure:
Standard image High-resolution imageConclusions
In summary, by doping Bi2O3 into the borosilicate glass matrix, the sintering temperature of the CsPbBr3 quantum dot precursor is reduced, and the precipitation of the CsPbBr3 quantum dot in the borosilicate glass matrix is realized after heat treatment. The XRD pattern and TEM image structure characterization proved that the CsPbBr3 quantum dot were indeed crystallized in the borosilicate glass matrix. It also proves that the doped Bi3+ ions only exist in the borosilicate glass matrix, but do not participate in the formation of CsPbBr3 quantum dot. By adjusting the concentration of doped Bi2O3, tunable emission from 523 to 493 nm can be achieved. Finally, the CsPbBr3:xBi3+ (x = 0.06) sample was tested for cold and hot cycling and cold and hot impact. The test results showed that the prepared sample had good thermal stability, which provided a good solution for solving the problem of poor thermal stability of perovskite quantum dot.
Acknowledgments
This work was supported by Science and Technology Planning Project of Zhejiang Province, China (2018C01046), Enterprise-funded Latitudinal Research Projects (J2017-171; J2017-293 and J2017-243), Sponsored by Shanghai Sailing Program (18YF1422500) and Research start-up project of Shanghai Institute of Technology (YJ2018-9).







