Abstract
Purpose
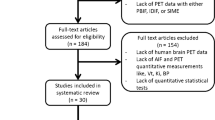
Preclinical dynamic brain PET studies remain hampered by the limitations related to the measurement of the arterial input function (AIF). In this regard, the use of an arterial-venous shunt is a promising method for the generation of real-time AIFs, but its application in longitudinal studies is still impeded by the cumbersome surgeries and high failure rates. We studied the feasibility and reproducibility of double arterial-venous shunt strategies for conducting longitudinal PET studies with real-time AIFs in rats.
Procedures
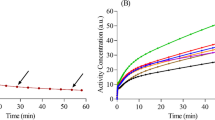
We studied the feasibility of double arterial-venous shunts in rats in the right/left inguinal region and evaluated inter-animal and intra-animal AIF reproducibilities. Image-derived input function (IDIF) was also obtained for comparison. Dynamic brain FDG PET studies were conducted to estimate kinetic constants and Cerebral Metabolic Rate of Glucose (CMRglc) obtained from standard 2-tissue compartment (2TCM) and Patlak analysis.
Results
We showed that longitudinal AIFs from double arterial-venous shunts can be obtained with very high success rate of the surgeries (88 %). Our results provided highly reproducible AIF measurements with low inter-animal variabilities (11 %) and intra-animal variabilities (5–10 %) that were included into the kinetic models, such that longitudinal rate constants and CMRglc can be efficiently estimated without bias associated to the double shunt. Our results indicated that longitudinal IDIF can be also generated without bias along time but showing higher intra-animal uncertainties.
Conclusions
We have demonstrated the feasibility and high reproducibility of conducting longitudinal AIF measurements and consequently accurate kinetic modeling using arterial shunt method.






Similar content being viewed by others
References
Sokoloff L, Reivich M, Kennedy C et al (1977) The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Patlak CS, Blasberg RG, Fenstermacher JD (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3(1):1–7
Alf MF, Martić-Kehl MI, Schibli R, Krämer SD (2013) FDG kinetic modeling in small rodent brain PET: optimization of data acquisition and analysis. Eur J Nucl Med Mol I 3:61
Weber B, Burger C, Biro P, Buck A (2002) A femoral arteriovenous shunt facilitates arterial whole blood sampling in animals. Eur J Nucl Med Mol Imaging 29:319–323
Pain F, Lanièce P, Mastrippolito R, Gervais P, Hantraye P, Besret L (2004) Arterial input function measurement without blood sampling using a beta-microprobe in rats. J Nucl Med 45(9):1577–1582
Fang YH, Muzic RF Jr (2008) Spillover and partial-volume correction for image-derived input functions for small-animal 18F-FDG PET studies. J Nucl Med 49(4):606–614
Zanotti-Fregonara P, Chen K, Liow JS, Fujita M, Innis RB (2011) Image-derived input function for brain PET studies: many challenges and few opportunities. J Cereb Blood Flow Metab 31(10):1986–1998
Wu HM, Sui G, Lee CC, Prins ML, Ladno W, Lin HD et al (2007) In vivo quantitation of glucose metabolism in mice using small-animal PET and a microfluidic device. J Nucl Med 48(5):837–845
Convert L, Morin-Brassard G, Cadorette J, Archambault M, Bentourkia M, Lecomte R (2007) A new tool for molecular imaging: the microvolumetric beta blood counter. J Nucl Med 48:1197–1206
Weber B, Spath N, Wyss M et al (2003) Quantitative cerebral blood flow measurements in the rat using a beta-probe and H2 15O. J Cereb Blood Flow Metab 23:1455–1460
Alf MF, Wyss MT, Buck A, Weber B, Schibli R, Kramer SD (2013) Quantification of brain glucose metabolism by 18F-FDG PET with real-time arterial and image derived input function in mice. J Nucl Med 54:132–138
Roehrbacher F, Bankstahl JP, Bankstahl M et al (2015) Development and performance test of an online blood sampling system for determination of the arterial input function in rats. EJNMMI Phys 2:1–19
Warnock GI, Aerts J, Bahri MA, Bretin F, Lemaire C, Giacomelli F et al (2014) Evaluation of 18F-UCB-H as a novel PET tracer for synaptic vesicle protein 2A in the brain. J Nucl Med 55(8):1336–1341
Herde AM, Keller C, Sephton SM, Mu L, Schibli R, Ametamey SM et al (2015) Quantitative positron emission tomography of mGluR5 in rat brain with [(18) F]PSS232 at minimal invasiveness and reduced model complexity. J Neurochem 133(3):330–342
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160(7):1577–1579
Huang Q, Massey JC, Mińczuk K, Li J, Kundu BK (2019) Non-invasive determination of blood input function to compute rate of myocardial glucose uptake from dynamic FDG PET images of rat heart in vivo: comparative study between the inferior vena cava and the left ventricular blood pool with spill over and partial volume corrections. Phys Med Biol 64(16):165010
Pain F, Laniece P, Mastrippolito R et al (2000) SIC, an intracerebral radiosensitive probe for in vivo neuropharmacology investigations in small laboratory animals: theoretical considerations and practical characteristics. IEEE Trans Nucl Sci 47:25–32
Napieczynska H, Kolb A, Katiyar P, Tonietto M, Ud-Dean M, Stumm R et al (2018) Impact of the arterial input function recording method on kinetic parameters in small-animal PET. J Nucl Med 59(7):1159–1164
Jespersen B, Knupp L, Northcott CA (2012) Femoral arterial and venous catheterization for blood sampling, drug administration and conscious blood pressure and heart rate measurements. J Vis Exp 59:3496
Huang CC, Wu CH, Huang YY, Tzen KY, Chen SF, Tsai ML, Wu HM (2017) Performing repeated quantitative small-animal pet with an arterial input function is routinely feasible in rats. J Nucl Med 58(4):611–616
Park M-J, Fung GS, Yamane T, Kaiser F, Fukushima K, Higuchi T (2013) Assessment of partial volume effect in small animal cardiac PET imaging using Monte Carlo simulation. J Nucl Med 54(suppl 2):1638
Kim SJ et al (2008) Kinetic modeling of 3’-Deoxy-3’ -18F-fluorothymidine for quantitative cell proliferation imaging in subcutaneous tumor models in mice. J Nucl Med 49:2057–2066
Fung EK, Carson RE (2013) Cerebral blood flow with [15O] water PET studies using an image-derived input function and MR-defined carotid centerlines. Phys Med Biol 58(6):1903–1923
Islam MM, Tsujikawa T, Mori T, Kiyono Y, Okazawa H (2017) Estimation of arterial input by a noninvasive image derived method in brain H215O PET study: confirmation of arterial location using MR angiography. Phys Med Biol 62(11):4514–4524
Deidda D, Karakatsanis NA, Robson PM et al (2019) Hybrid PET/MR kernelised expectation maximisation reconstruction for improved image-derived estimation of the input function from the aorta of rabbits. Contrast Media Mol Imaging 2019:3438093
Lanz B, Poitry-Yamate C, Gruetter R (2014) Image-derived input function from the vena cava for 18F-FDG PET studies in rats and mice. J Nucl Med 55(8):1380–1388
Thackeray JT, Bankstahl JP, Bengel FM (2015) Impact of image-derived input function and fit time intervals on Patlak quantification of myocardial glucose uptake in mice. J Nucl Med 56(10):1615–1621
Acknowledgments
This work was partially supported by the projects ED431F 2017/04 (Xunta de Galicia), EAPA_791/2018 (Interreg UE) and RYC-2015/17430 (Ramón y Cajal, Pablo Aguiar).
Author information
Authors and Affiliations
Contributions
DRB made the surgeries and wrote the manuscript; AM prepared the figures and contributed to the analysis of data; NGL verified the analytical methods and worked out almost all of the technical details; AFF and JSR helped analyze the results; AR helped design and instruct the experiment; PA conceived the study and revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rey-Bretal, D., Moscoso, A., Gómez-Lado, N. et al. Feasibility of Longitudinal Brain PET with Real-Time Arterial Input Function in Rats. Mol Imaging Biol 23, 350–360 (2021). https://doi.org/10.1007/s11307-020-01556-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01556-y