Abstract
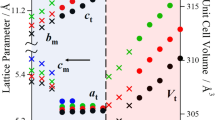
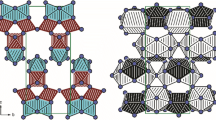
We report a series of novel alkali rare-earth orthoborates K3RE3(BO3)4 (RE = Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu) prepared by high-temperature solid-state syntheses. Single crystals of K3Pr3(BO3)4, K3Er3(BO3)4, K3Tm3(BO3)4, K3Yb3(BO3)4 and K3Lu3(BO3)4 are obtained by spontaneous crystallization using a flux. Complementary single-crystal and powder X-ray diffraction measurements followed by structure refinements reveal that both Pr- and Nd-containing phases crystallize in the space group P\(\overline{1}\) (Z = 4), whereas the other K3RE3(BO3)4 members with higher atomic number (RE = Sm–Lu) crystallize in the higher symmetry space group P21/c (Z = 8). Amid differences in the space groups, each member of the series keeps close structural correlations in their motif and connectivity. The crystal structures of K3RE3(BO3)4 (RE = Sm–Lu) consist of quasi-two-dimensional [RE8(BO3)8] layers parallel to the ab-plane which are connected by out-of-layer RE2O12 and RE2O14 dimers as well as BO3 groups in the c-direction, forming a 3D framework. On the other hand, both K3Pr3(BO3)4 and K3Nd3(BO3)4 phases are comprised of the [RE4(BO3)4] layers, indicating the absence of 21 screw axis along the b-axis. The K+ cations are located between the [RE8(BO3)8] and [RE4(BO3)4] layers, occupying the interstitial voids. All samples exhibit characteristic absorption features in the UV/Vis range relevant to the rare-earth cations, and their optical band gaps are evaluated by both conventional Tauc plot and derivation of absorption spectrum fitting (DASF) methods. The geometric deviations away from the D3h symmetry of the BO3 planar groups are verified using Fourier transformed infrared and Raman spectra, and supported by the X-ray diffraction results.






Similar content being viewed by others
References
Rao RP, Devine DJ (2000) RE-activated lanthanide phosphate phosphors for PDP applications. J Lumin 87–89:1260–1263
Rao RP (2003) Tb3+ activated green phosphors for plasma display panel applications. J Electrochem Soc 150:H165–H171
Zhang L, Pédrini C, Madej C, Dujardin C, Gcon JC, Moine B, Kamenskikh I, Belsky A, Shaw DA, MacDonald MA, Mesnard P, Fouassier C, Van’t Spijker JC, Van Eijk CWE (1999) Fast fluorescence and scintillation properties of cerium and praseodymium doped lutetium orthoborates. Radiat Eff Defects Solids 150:47–52
Zhang Z-J, Jin T-T, Xu M-M, Huang Q-Z, Li M-R, Zhao J-T (2015) Low-temperature vaterite-type LuBO3, a vacancy-stabilized phase synthesized at high temperature. Inorg Chem 54:969–975
Gheorghe L, Greculeasa M, Broasca A, Voicu F, Stanciu G, Belikov KN, Bryleva EY, Gaiduk O (2019) Incongruent melting LaxYySc4-x-y(BO3)4: LYSB nonlinear optical crystal grown by the Czochralski method. ACS Appl Mater Interfaces 11:20987–20994
Aka G, Reino E, Loiseau P, Vivien D, Ferrand B, Fulbert L, Pelenc D, Lucas-Leclin G, Georges P (2004) Ca4REO(BO3)3 crystals for green and blue microchip laser generation: from crystal growth to laser and nonlinear optical properties. Opt Mater 26:431–436
Zou G, Ma Z, Wu K, Ye N (2012) Cadmium-rare earth oxyborates Cd4ReO(BO3)3 (Re = Y, Gd, Lu): congruently melting compounds with large SHG responses. J Mater Chem 22:19911–19918
Rytz D, Gross A, Vernay S, Wesemann V (2008) YAl3(BO3)4: a novel NLO crystal for frequency conversion to UV wavelengths, Proc. SPIE, Solid State Lasers Amplifiers III, 6998
Yue Y, Zhu Y, Zhao Y, Tu H, Hu Z (2016) Growth and nonlinear optical properties of GdAl3(BO3)4 in a flux without molybdate. Cryst Growth Des 16:347–350
Fang SH, Liu H, Huang LX, Ye N (2013) Growth and optical properties of nonlinear LuAl3(BO3)4 crystals. Opt Express 21:16415–16423
Mutailipu M, Xie Z, Su X, Zhang M, Wang Y, Yang Z, Janjua M, Pan S (2017) Chemical cosubstitution-oriented design of rare-earth borates as potential ultraviolet nonlinear optical materials. J Am Chem Soc 139:18397–18405
Xie Z, Mutailipu M, He G, Han G, Wang Y, Yang Z, Zhang M, Pan S (2018) A series of rare-earth borates K7MRE2B15O30 (M = Zn, Cd, Pb; RE = Sc, Y, Gd, Lu) with large second harmonic generation responses. Chem Mater 30:2414–2423
Gorbachenya KN, Kisel VE, Yasukevich AS, Maltsev VV, Leonyuk NI, Kuleshov NV (2013) Highly efficient continuous-wave diode-pumped Er, Yb:GdAl3(BO3)4 laser. Opt Lett 38:2446–2448
Broasca A, Gheorghe L, Greculeasa M, Voicu F, Stanciu G, Hau S, Gheorghe C, Croitoru G, Pavel N, (2019) Czochralski Growth and Characterization of Pure and Yb-Doped LaxYySc4-x-y(BO3)4 Nonlinear and Laser Crystal, Laser Applications Conference, Opt. Soc. Am, pp. JM5A. 40
Dekker P, Dawes JM, Piper JA, Liu Y, Wang J (2001) 1.1 W CW self-frequency-doubled diode-pumped Yb:YAl3(BO3)4 laser. Opt Comm 195:431–436
Lu DZ, Fang QN, Yu XS, Han XK, Wang JY, Yu HH, Zhang HJ (2019) Power scaling of the self-frequency-doubled quasi-two-level Yb:YCOB laser with a 30% slope efficiency. Opt Lett 44:5157–5160
Li RK, Wu CC, Xia MJ (2016) LiCaTb5(BO3)6: a new magneto-optical crystal promising as Faraday rotator. Opt Mater 62:452–457
Li R (2019) Enhancing the magnetocaloric effect of a paramagnet to above liquid hydrogen temperature. Energy Technol 7:1801070
Abdullaev GK, Mamedov KS, Amiraslanov IR, Magerramov AI (1977) Crystal structure of lithium praseodymium orthoborate Li3Pr2(BO3)3. J Struct Chem 18:331–333
Sebastian B, Hubert H (2017) Synthesis and structural characterization of Li3Y(BO3)2. Z Naturforsch B 72:153–158
Jubera V, Gravereau P, Chaminade JP (2001) Crystal structure of the new borate Li3Gd(BO3)2. Comparison with the homologous Na3Ln(BO3)2 (Ln: La, Nd) compounds. Solid State Sci 3:469–475
Mascetti J, Vlasse M, Fouassier C (1981) The crystal chemistry of the new rare-earth sodium borates Na3Ln(BO3)2(Ln = La, Nd). J Solid State Chem 39:288–293
Zhang Y, Chen XL, Liang JK, Xu T (2002) Synthesis and structural study of new rare earth sodium borates Na3Ln(BO3)2 (Ln=Y, Gd). J Alloys Compd 333:72–75
Wang Z, Li H, Cai G, Jin Z (2016) Synthesis, crystal structure, and thermal stability of new borates Na3REB2O6 (RE = Pr, Sm, Eu). Powder Diffr 31:110–117
Shan F, Kang L, Zhang G, Yao J, Lin Z, Xia M, Zhang X, Fu Y, Wu Y (2016) Na3Y3(BO3)4: a new noncentrosymmetric borate with an open-framework structure. Dalton Trans 45:7205–7208
Gao JH, Li RK (2008a) Potassium rich rare earth (RE) borates K3RE(BO3)2. Solid State Sci 10:26–30
Zhao S, Zhang G, Yao J, Wu Y (2012) K3YB6O12: a new nonlinear optical crystal with a short UV cutoff edge. Mater Res Bull 47:3810–3813
Gao JH, Li RK (2008b) Preparation, structure and luminescent properties of a new potassium yttrium borate K3Y3(BO3)4. Mate Res Bull 43:882–888
Atuchin VV, Subanakov AK, Aleksandrovsky AS, Bazarov BG, Bazarova JG, Dorzhieva SG, Gavrilova TA, Krylov AS, Molokeev MS, Oreshonkov AS, Pugachev AM, Tushinova YL, Yelisseyev AP (2017) Exploration of structural, thermal, vibrational and spectroscopic properties of new noncentrosymmetric double borate Rb3NdB6O12. Adv Powder Technol 28:1309–1315
Subanakov AK, Kovtunets EV, Bazarov BG, Dorzhieva SG, Bazarova JG (2020) New double holmium borates: Rb3HoB6O12 and Rb3Ho2B3O9. Solid State Sci 105:106231
Atuchin VV, Subanakov AK, Aleksandrovsky AS, Bazarov BG, Bazarova JG, Gavrilova TA, Krylov AS, Molokeev MS, Oreshonkov AS, Stefanovich SY (2018) Structural and spectroscopic properties of new noncentrosymmetric self-activated borate Rb3EuB6O12 with B5O10 units. Mater Design 140:488–494
Gao JH, Zheng MS (2009) Synthesis and Crystal Structure of a Rubidium Lanthanum Borate Rb3La3(BO3)4. Chin J Struct Chem 28:82–86
Sheldrick G (2015) SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8
Hubschle CB, Sheldrick GM, Dittrich B (2011) ShelXle: a Qt graphical user interface for SHELXL. J Appl Crystallogr 44:1281–1284
Spek A (2003) Single-crystal structure validation with the program PLATON. J Appl Crystallogr 36:7–13
Rodriguez-Carvajal J, Roisnel T, (2000) WinPLOTR: A windows tool for powder diffraction pattern analysis, Mater. Sci. Forum, proceedings of the seventh European powder diffraction conference, Barcelona, Spain, pp. 378–381.
Kubelka P (1931) Ein Beitrag zur Optik der Farbanstriche. Z Tech Phys 12:593–601
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi B 15:627–637
Souri D, Tahan ZE (2015) A new method for the determination of optical band gap and the nature of optical transitions in semiconductors. Appl Phys B 119:273–279
Kirsch A, Murshed MM, Schowalter M, Rosenauer A, Gesing TM (2016) Nanoparticle precursor into polycrystalline Bi2Fe4O9: an evolutionary investigation of structural, morphological, optical, and vibrational properties. J Phys Chem C 120:18831–18840
Kirsch A, Murshed MM, Litterst FJ, Gesing TM (2019) Structural, spectroscopic, and thermoanalytic studies on Bi2Fe4O9: tunable properties driven by nano-and poly-crystalline states. J Phys Chem C 123:3161–3171
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–767
Jia YQ (1991) Crystal radii and effective ionic radii of the rare earth ions. J Solid State Chem 95:184–187
Lin JH, Su MZ, Wurst K, Schweda E (1996) The structure of La26(BO3)8O27: a structure with a distorted fluorite type arrangement of atoms. J Solid State Chem 126:287–291
Noirault S, Célérier S, Joubert O, Caldes MT, Piffard Y (2007a) Effects of water uptake on the inherently oxygen-deficient compounds Ln26O27□(BO3)8 (Ln = La, Nd). Inorg Chem 46:9961–9967
Noirault S, Célérier S, Joubert O, Caldes MT, Piffard Y (2007b) Incorporation of Water and fast proton conduction in the inherently oxygen-deficient compound La26O27□(BO3)8. Adv Mater. 19:867–870
Dieke GH, Crosswhite HM (1963) The spectra of the doubly and triply ionized rare earths. Appl Opt 2:675–686
Carnall WT, Fields PR, Rajnak K (1968) Electronic energy levels in the trivalent lanthanide Aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J Chem Phys 49:4424–4442
Prokofiev AV, Shelykh AI, Melekh BT (1996) Periodicity in the band gap variation of Ln2X3 (X = O, S, Se) in the lanthanide series. J Alloys Compd 242:41–44
He R, Huang H, Kang L, Yao W, Jiang X, Lin Z, Qin J, Chen C (2013) Bandgaps in the deep ultraviolet borate crystals: prediction and improvement. Appl Phys Lett 102:231904
Haberer A, Kaindl R, Huppertz H (2010) Synthesis and crystal structure of the praseodymium orthoborate λ -PrBO3. Z Naturforsch B 65:1206–1212
Nakamoto K (2006) Infrared and raman spectra of inorganic and coordination compounds. In: Handbook of vibrational spectroscopy. Wiley-Interscience. https://doi.org/10.1002/0470027320.s4104
Tao C, Wu C, Xia M, Li R (2019) Li2Ca5Tb(BO3)5: An orthoborate with large spherical hollow cages. Opt Mater 96:109358
Acknowledgements
PYC gratefully thanks the Chinese Scholarship Council (CSC) for the financial support. The University of Bremen is cordially acknowledged for the technical and administrative supports. The authors thank the Deutsche Forschungsgemeinschaft (DFG) for financial supports for large scientific instruments INST 144/458-1 FUGG used for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Shen Dillon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, P., Murshed, M.M. & Gesing, T.M. Synthesis and crystal structures of novel alkali rare-earth orthoborates K3RE3(BO3)4 (RE = Pr, Nd, Sm–Lu). J Mater Sci 56, 3639–3652 (2021). https://doi.org/10.1007/s10853-020-05506-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05506-5