Abstract
The medaka Oryzias latipes is a teleost fish with an XX/XY sex determination system similar to that of mammals. However, under high-temperature conditions, XX medaka are masculinized by an elevation of the key teleost glucocorticoid, cortisol. Cortisol inhibits female-type proliferation of germ cells and induces masculinization of XX medaka during gonadal sex differentiation. To identify masculinization mechanisms downstream of cortisol, we analysed the functions of gonadal soma-derived growth factor (gsdf) and anti-Müllerian hormone receptor type 2 (amhr2); these genes are known to play important roles in the inhibition of germ cell proliferation and male differentiation. We investigated the impact of gsdf and amhr2 on the proliferation of germ cells using gsdf knockout (KO) and amhr2 KO medaka. At hatching stage, loss of gsdf or amhr2 function recovered female-type proliferation in germ cells under cortisol treatment. Moreover, cortisol treatment of gsdf KO or amhr2 KO medaka did not induce masculinization of XX medaka. These results suggest that cortisol inhibits female-type proliferation of germ cells and induces masculinization of XX medaka through GSDF and AMHR2. This study thereby provides the first evidence that GSDF and AMHR2 are involved in cortisol-induced masculinization.
Similar content being viewed by others
Introduction
Many vertebrates reproduce sexually and have separate male and female sexes determined by genotype; however, sex determination in poikilothermic vertebrates—such as reptiles, amphibians, and fish—is also highly influenced by ambient temperature (Baroiller et al. 1999). Furthermore, in some teleost fishes, sex can be affected by environmental variables such as pH (Rubin 1985), density (Francis 1984), and social factors (Francis and Barlow 1933). The molecular mechanisms of environmental sex determination in such species are not well understood.
The medaka Oryzias latipes is a small gonochoristic teleost fish that offers many advantages for researchers, including the availability of a range of useful strains (Ishikawa 2000). Applications of transgenic techniques and gene knockout (KO) systems, using transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9), have also been established in medaka (Ozato et al. 1986; Ansai et al. 2013; Sawamura et al. 2017). Medaka has an XX/XY sex determination system, and the sex-determining gene dmy (also known as dmrt1bY) has been identified on the medaka Y chromosome (Matsuda et al. 2002). Medaka is therefore a suitable model vertebrate for the genetic analysis of sex determination and differentiation. The first appearance of morphological sex differentiation in medaka is a difference in the number of germ cells between sexes prior to hatching. Germ cells in genetic females (XX) proliferate rapidly and subsequently initiate oogenesis, while germ cells in genetic males (XY) remain quiescent (Satoh and Egami 1972; Kobayashi et al. 2004). Notably, exposure to high-temperature (HT) treatment during sex differentiation inhibits germ cell proliferation and induces masculinization in XX medaka (Sato et al. 2005; Hattori et al. 2007; Selim et al. 2009). HT also induces masculinization by elevating the levels of cortisol, which is the major glucocorticoid in teleost fish (Hayashi et al. 2010). In fish, cortisol is secreted from interrenal tissues by environmental stimuli such as acidic water and rapid temperature changes (Wendelaar Bonga 1997). The frequencies of XX males caused by cortisol treatment varies depending on experimental conditions including treatment periods and medaka strains (21.9–95.7%) (Hayashi et al. 2010; Kitano et al. 2012; Adolfi et al. 2019; Hara et al. 2020). Moreover, exposure to cortisol or HT in XX medaka is also known to elevate the expression of gonadal soma-derived growth factor (gsdf) by 5 days post-hatching (dph) (Kitano et al. 2012); however, the exact role of HT-elevated cortisol in the masculinization of XX medaka remains unknown.
GSDF plays an essential role in the development of both the testis and the ovary, as demonstrated by gain- and loss-of-function experiments in medaka (Zhang et al. 2016). The expression of gsdf co-localizes with dmy, and gsdf is regulated by dmy during the initiation of morphological testicular differentiation (Shibata et al. 2010; Zhang et al. 2016). Importantly, gsdf depletion leads to an excessive proliferation of germ cells during sexual differentiation regardless of sex, and some XY gonads in gsdf mutants develop into ovaries at the adult stage (Imai et al. 2015; Zhang et al. 2016). These phenotypes resemble those of anti-Müllerian hormone receptor 2 (amhr2) KO medaka (Morinaga et al. 2007). Anti-Müllerian hormone (AMH) signalling regulates proliferation at a specific stage of germ cell development, and this regulation is crucial for the proper manifestation of gonadal sex directed by sex determination genes (Nakamura et al. 2012; Pfennig et al. 2015). Thus, the importance of gsdf and amhr2 in sexual development has been established; however, our understanding of how these genes function in cortisol-induced masculinization is limited.
In this study, we investigated whether GSDF and AMHR2 are involved in masculinization by cortisol. First, we generated gsdf KO medaka using the CRISPR/Cas9 system. Then, we investigated the number of germ cells at the hatching stage in the gsdf KO medaka, and in previously generated amhr2 KO medaka (Furukawa et al. 2019), following treatment with cortisol. We also examined the sex ratios of gsdf KO and amhr2 KO adult fish by histological observation of the gonads after cortisol treatment.
Materials and methods
Ethics statement
The study was performed using protocols approved by the Animal Care and Use Committee of Kumamoto University (Approval number: 30-022). All experiments were performed in accordance with the relevant guidelines and regulations.
Animals
Female leucophore-free (FLFII) medaka stock was used (Wakamatsu et al. 2003); this stock allows the identification of genotypic sex before the onset of sex differentiation, via the appearance of leucophores at 2 days post-fertilization (dpf). Fish embryos and larvae were maintained in embryo rearing medium (ERM) (17 mM NaCl, 0.4 mM KCl, 0.27 mM CaCl2 2H2O, 0.66 mM MgSO4, pH 7), at a water temperature of 26 °C, with a 14 h light and 10 h dark cycle.
Generation of gsdf KO medaka
Synthetic CRISPR RNAs (crRNA) and trans-activating crRNA (tracrRNA) were obtained from FASMAC Co. (Kanagawa, Japan), and recombinant Cas9 protein was obtained from PNA Bio Inc. (Thousand Oaks, CA, USA); these were used as previously described (Sawamura et al. 2017). The sequences of the two synthetic crRNAs for gsdf were as follows:
CAUGGAGCAGCACUUCAGGCguuuuagagcuaugcuguuuug,
CGCGGCUGCGGCUGUGGACCguuuuagagcuaugcuguuuug.
Microinjection was performed on one-cell embryos using a Nanoject II (Drummond Scientific Co., Broomall, PA, USA). Cas9 protein (500 ng/μL), tracrRNA (200 ng/μL), and the two crRNAs (100 ng/μL) were simultaneously injected into embryos. After injection, the embryos were maintained in ERM at 26 °C.
For genotyping, genomic DNA was extracted from adult caudal fins, as described previously (Hayashi et al. 2010). Genetic sex was then determined by genomic polymerase chain reaction (PCR) using primers specific for dmy, as also described previously (Hayashi et al. 2010). The gsdf genotypes were determined by PCR using primers specific for gsdf (5′-CATGGGTGTCACGGACACTAC-3′ and 5′-ATCAGCCTCACAGGTGTTTG-3′) and AmpliTaq Gold (Applied Biosystems). The PCR conditions were as follows: preheating at 95 °C for 10 min, 40 cycles of PCR at 94 °C for 30 s, 59 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 5 min. PCR products were subcloned into the pT7Blue vector (Novagen) and sequenced with the GenomeLab GeXP™ Genetic Analysis System (Beckman Coulter, Brea, CA, USA).
Experimental treatment
Cortisol treatments were performed by rearing fish with hydrocortisone (5 × 10–6 M; Sigma-Aldrich, Gillingham, UK) from 0 dpf to 5 dph, as previously described (Hayashi et al. 2010; Kitano et al. 2012; Hara et al. 2020). After treatment, fish were maintained up to adulthood (2 months post-hatching; mph) at 26 °C.
Histological analysis
Fish at 0 dph and 2 mph were fixed in Bouin’s solution overnight at 4 °C, then dehydrated, embedded in paraffin, and serially sectioned at 5 μm, as described previously (Hayashi et al. 2010). Sections were stained with haematoxylin and eosin as also described previously (Hayashi et al. 2010). Germ cells were counted using an MZ FLIII microscope (Leica Microsystems).
Statistical analysis
Experimental results were tested using Levene’s test for homogeneity of variance. Data were analysed by Student’s t-test or by one-way ANOVA followed by Tukey’s multiple comparison test using SPSS statistics 20 (IBM Corp., Armonk, NY).
Results
Generation of gsdf KO medaka
We knocked out medaka gsdf using the CRISPR/Cas9 system (Sawamura et al. 2017). As shown in Fig. 1, deletion of the transforming growth factor-β (TGF-β) domain in gsdf was achieved by simultaneous injection of two crRNAs, a tracrRNA, and the Cas9 protein into 1-cell stage embryos. After mating injected medaka with wild-type fish, gsdf genotypes in the F1 generation were determined by sequence analysis of PCR products amplified from genomic DNA extracted from adult caudal fins. The gsdf KO fish were obtained by crossing F1 heterozygous mutants.
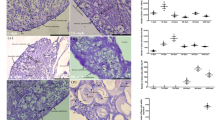
Number of germ cells at the hatching stage in cortisol-treated gsdf KO and amhr2 KO medaka
We confirmed the effects of cortisol on gsdf KO and amhr2 KO medaka (Furukawa et al. 2019). First, we counted the number of germ cells at the hatching stage in medaka treated with cortisol from 0 dpf. In wild-type fish, XX individuals treated with cortisol had around 50 germ cells, which corresponds to the number of germ cells in males. In contrast, there were high numbers of germ cells in gsdf KO medaka and amhr2 KO medaka, in both XX and XY individuals, with and without cortisol treatment (Fig. 2); gsdf KO XX medaka with and without cortisol treatment had significantly more germ cells than wild-type XX fish at the hatching stage.
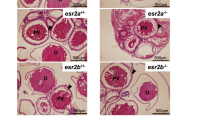
Sex ratios in cortisol-treated gsdf KO and amhr2 KO medaka
We examined the sex ratios of adult fish by histological observation of the gonads at 2 mph after treatment with cortisol during the sexual differentiation period. In wild-type fish, cortisol treatment induced masculinization of XX individuals in some cases (Fig. 3a–e, Table 1). Conversely, gsdf KO medaka and amhr2 KO medaka were not masculinized by cortisol. In addition, under non-cortisol conditions, ovaries were found in several XY individuals of the KO medaka (Fig. 3f–o, Table 1). Histological examination revealed testes or ovaries only; there were no cases of intersex gonads.
Discussion
To investigate whether gsdf is essential for cortisol-induced masculinization, we knocked out the TGF-β domain of medaka gsdf using the CRISPR/Cas9 system (Sawamura et al. 2017). The gsdf KO medaka underwent female-type proliferation of germ cells, and 7.1% of XY gsdf KO fish differentiated into females. In addition, gsdf KO females had larger ovaries than wild-type females and were fertile (data not shown). As these data are similar to previously reported phenotypes of gsdf KO medaka (Imai et al. 2015; Zhang et al. 2016; Guan et al. 2017) in which the location of the gsdf mutation differed from that in our study, we inferred that the gsdf KO line generated here also lacks gsdf function.
In this study, we showed that cortisol induces masculinization of wild-type XX medaka, but not of gsdf KO and amhr2 KO XX fish, suggesting important roles of GSDF and AMHR2 in cortisol-induced masculinization. The frequency of wild-type XX males induced by cortisol in this study was lower than that in previous reports (Hayashi et al. 2010; Kitano et al. 2012; Adolfi et al. 2019; Hara et al. 2020). This may be due to the death of the masculinized fish during breeding, because the final survival rate (48.9%) of the cortisol-treated individuals in this study was lower than that (59.0%) in a recent study (Hara et al. 2020). On the other hand, cortisol inhibited female-type proliferation of germ cells at hatching stage in wild-type XX medaka, but not in gsdf KO and amhr2 KO XX fish. This suggests that cortisol inhibits germ cell proliferation via gsdf and amhr2. These results indicate that GSDF and AMHR2 are indispensable for cortisol-induced masculinization and male-type proliferation of germ cells.
All gsdf KO and amhr2 KO XY medaka showed a female-type proliferation of germ cells at hatching stage. However, as also seen previously, some gsdf KO and amhr2 KO XY medaka developed into males by adulthood, under non-cortisol treatment (Imai et al. 2015; Zhang et al. 2016; Morinaga et al. 2007). It is thought that doublesex- and mab-3-related transcription factor 1 (dmrt1), which acts downstream of gsdf, affects the differentiation of gsdf KO XY medaka into males (Imai et al. 2015). It was recently reported that the expression of dmrt1 was dramatically down-regulated in gsdf KO XY gonads compared with wild-type XY gonads, indicating that GSDF may directly or indirectly activate or maintain its expression (Zhang et al. 2016). Moreover, exposure to cortisol or HT in XX medaka induced dmrt1 expression during sex differentiation (Adolfi et al. 2019). Therefore, cortisol appears to activate and maintain masculinization of XX medaka by inducing the expression of dmrt1 via GSDF.
Our previous study provided evidence that peroxisome proliferator-activated receptor α (PPARα) is involved in masculinization by cortisol (Hara et al. 2020). Interestingly, PPARα agonist treatment induced masculinization of XX medaka in some cases. In addition, pparaa KO XX medaka were not masculinized by cortisol, as observed in gsdf KO and amhr2 KO medaka in this study, suggesting that activation of PPARα may directly or indirectly regulate gsdf and amhr2 expression (Fig. 4). Currently, little is known about the relationship between PPARα and male-promoting genes. To elucidate the masculinization mechanism, it will be necessary to investigate whether PPARα directly induces the expression of gsdf and amhr2, for example, by the analysis of DNA–protein complexes via chromatin immunoprecipitation assays.
In summary, this study provides the first evidence that GSDF and AMHR2 are involved in cortisol-induced masculinization in medaka. Elucidation of the molecular mechanisms by which this stress hormone affects sex is expected to greatly contribute to artificial sex control. In medaka, gsdf and amhr2 are located on autosomal chromosomes, but in some fish species these genes are sex-determining genes: for example, gsdf in Oryzias luzonensis (Myosho et al. 2012), and amhr2 in Takifugu rubripes (Kamiya et al. 2012). This suggests that genetic female fish of such species have lost the masculinization functions of gsdf or amhr2. Furthermore, if gsdf or amhr2 are essential for masculinization by cortisol in fish, then cortisol may not be able to induce masculinization of genetic females in these species. Future studies will therefore focus on the effects of environmental stress or cortisol on fish species in which gsdf and amhr2 are sex-determining genes.
Change history
04 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12562-022-01609-x
References
Adolfi MC, Fischer P, Herpin A, Regensburger M, Kikuchi M, Tanaka M, Schartl M (2019) Increase of cortisol levels after temperature stress activates dmrt1a causing female-to-male sex reversal and reduced germ cell number in medaka. Mol Reprod Dev 86:1405–1417
Ansai S, Sakuma T, Yamamoto T, Ariga H, Uemura N, Takahashi R, Kinoshita M (2013) Efficient targeted mutagenesis in medaka using custom-designed transcription activator-like effector nucleases. Genetics 193:739–749
Baroiller JF, Guiguen Y, Fostier A (1999) Endocrine and environmental aspects of sex differentiation in fish. Cell Mol Life Sci 55:910–931
Francis RC (1984) The effects of bidirectional selection for social dominance on agonistic behavior and sex ratios in the paradise fish (Macropodus opercularis). Behaviour 90:25–45
Francis RC, Barlow GW (1933) Social control of primary sex differentiation in the Midas cichlid. Proc Natl Acad Sci USA 90:10673–10675
Furukawa F, Hamasaki S, Hara S, Uchimura T, Shiraishi E, Osafune N, Takagi H, Yazawa T, Kamei Y, Kitano T (2019) Heat shock factor 1 protects germ cell proliferation during early ovarian differentiation in medaka. Sci Rep 9:6927
Guan G, Sun K, Zhang X, Zhao X, Li M, Yan Y, Wang Y, Chen J, Yi M, Hong Y (2017) Developmental tracing of oocyte development in gonadal soma-derived factor deficiency medaka (Oryzias latipes) using a transgenic approach. Mech Dev 143:53–61
Hara S, Furukawa F, Mukai K, Yazawa T, Kitano T (2020) Peroxisome proliferator-activated receptor alpha is involved in the temperature-induced sex differentiation of a vertebrate. Sci Rep 10:1–11
Hattori RS, Gould RJ, Fujioka T, Saito T, Kurita J, Strüssmann CA, Yokota M, Watanabe S (2007) Temperature-dependent sex determination in Hd-rR medaka Oryzias latipes: gender sensitivity, thermal threshold, critical period, and DMRT1 expression profile. Sex Dev 1:138–146
Hayashi Y, Kobira H, Yamaguchi T, Shiraishi E, Yazawa T, Hirai T, Kamei Y, Kitano T (2010) High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol Reprod Dev 77:679–686
Imai T, Saino K, Matsuda M (2015) Mutation of Gonadal soma-derived factor induces medaka XY gonads to undergo ovarian development. Biochem Biophys Res Commun 467:109–114
Ishikawa Y (2000) Medakafish as a model system for vertebrate developmental genetics. BioEssays 22:487–495
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K (2012) A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet 8:e1002798
Kitano T, Hayashi Y, Shiraishi E, Kamei Y (2012) Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature. Mol Reprod Dev 79:719–726
Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y (2004) Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev Dyn 231:518–526
Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N (2002) DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417:559–563
Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, Shimizu N, Mitani H, Furutani-Seiki M, Tanaka M, Kondoh H (2007) The hotei mutation of medaka in the anti-Müllerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc Natl Acad Sci USA 104:9691–9696
Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, Naruse K, Hamaguchi S, Sakaizumi M (2012) Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 191:163–170
Nakamura S, Watakabe I, Nishimura T, Picard JY, Toyoda A, Taniguchi Y, di Clemente N, Tanaka M (2012) Hyperproliferation of mitotically active germ cells due to defective anti-Müllerian hormone signaling mediates sex reversal in medaka. Development 139:2283–2287
Ozato K, Kondoh H, Inohara H, Iwamatsu T, Wakamatsu Y, Okada TS (1986) Production of transgenic fish: introduction and expression of chicken delta-crystallin gene in medaka embryos. Cell Differ 19:237–244
Pfennig F, Standke A, Gutzeit HO (2015) The role of Amh signaling in teleost fish–multiple functions not restricted to the gonads. Gen Comp Endocrinol 223:87–107
Rubin DA (1985) Effect of pH on sex ratio in cichlids and a poecilliid (Teleostei). Copeia 1985:233–235
Sato T, Endo Y, Yamahira K, Hamaguchi S, Sakaizumi M (2005) Induction of female-to-male sex reversal by high temperature treatment in medaka, Oryzias latipes. Zoolog Sci 22:985–988
Satoh N, Egami N (1972) Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development. J Embryol Exp Morphol 28:385–395
Sawamura R, Osafune N, Murakami T, Furukawa F, Kitano T (2017) Generation of biallelic F0 mutants in medaka using the CRISPR/Cas9 system. Genes Cells 22:756–763
Selim KM, Shinomiya A, Otake H, Hamaguchi S, Sakaizumi M (2009) Effects of high temperature on sex differentiation and germ cell population in medaka, Oryzias latipes. Aquaculture 28:340–349
Shibata Y, Paul-Prasanth B, Suzuki A, Usami T, Nakamoto M, Matsuda M, Nagahama Y (2010) Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expr Patterns 10:283–289
Wakamatsu Y, Inoue C, Hayashi H, Mishima N, Sakaizumi M, Ozato K (2003) Establishment of new medaka (Oryzias latipes) stocks carrying genotypic sex markers. Environ Sci 10:291–302
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Zhang X, Guan G, Li M, Zhu F, Liu Q, Naruse K, Herpin A, Nagahama Y, Li J, Hong Y (2016) Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci Rep 6:19738
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number 19H03052 (to T.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Gemma Richards, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hara, S., Sawamura, R. & Kitano, T. Cortisol induces masculinization of XX medaka through gonadal soma-derived growth factor (GSDF) and anti-Müllerian hormone receptor type 2 (AMHR2). Fish Sci 87, 85–91 (2021). https://doi.org/10.1007/s12562-020-01479-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01479-1