Abstract
Traditional cell culture makes use of two-dimensional (2-D) surfaces for unnatural characteristics in vitro cell response and growth. To overcome the limitation, 3-D culture models mimicking the cancer microenvironment in vivo are gaining attention. Herein, we investigated an improved composite 3-D biomimetic structure comprising poly(hydroxybutyrate-co-hydroxyvalerate) and carboxymethylcellulose (CMC) (PHBV-co-CMC) for determining the potentiation effects of Centella asiatica extract on lung cancer cells (A549). To improve its biological acceptance characteristics, the 3-D scaffold was infused with low viscosity CMC gel (0.009 ± 0.0004 Pa s) under vacuum pressure. The composite material was left immersed in complete growth media with the presence of cells to determine its optimum day of degradation prior to anti-proliferative (IC50) analysis through 2-D and 3-D cell culture. All scaffolds were found to be sustained for up to 7 days of incubation with breakages ranging from 10 to 70% (w/w). The A549 cell mortality treated with the extract (IC50 5.75 ± 1.0 µg/ml) on both porous 3-D PHBV and composite-CMC scaffolds were 70% higher than the 2-D model (30%) (p < 0.05). Visual assessment on the inner structure of the 3-D scaffold using fluorescent microscopy and scanning electron microscopy showed a scattering number of living cells on the scaffold pores wall and on the CMC gel with a glowing blue color indicating the presence of a livable cells post DAPI dye staining. Therefore, the structure of composite 3-D PHBV scaffold co-CMC produced a potential alternative method of a 3-D cell culture model to study the effectiveness of medicinal plant extracts (e.g. anticancer properties) in which might potentially mimic specific cancerous tissue ex vivo.






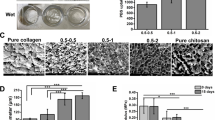
taken from a random sampling. Magnification ranges from × 40 to × 400

taken from a random sampling. Magnification ranges from × 80 to × 180

taken from a random sampling. Magnification ranges from × 200 to × 1200

taken from a random sampling. Magnification ranges from × 200 to × 1200


Similar content being viewed by others
Data Availability
No data were used elsewhere to support this study and it was entirely a new set of data.
References
Inamdar, P.K.; Yeole, R.D.; Ghogare, A.B.; De Souza, N.J.: Determination of biologically active constituents in Centella asiatica. J. Chromatogr. A 42(1), 127–130 (1997)
Babu, T.D.; Kuttan, G.; Padikkala, J.: Cytotoxic and anti-tumour properties of certain taxa of Umbelliferae with special reference to Centella asiatica (L.) Urban. J. Ethnopharmacol. 48(1), 53–57 (1995)
Mutua, P.M.; Gicheru, M.M.; Makanya, A.N.; Kiama, S.G.: Anti-proliferative activities of Centella asiatica extracts on human respiratory epithelial cells in vitro. Int. J. Morphol. 31(4), 1322–1327 (2013)
Yoshida, M.; Fuchigami, M.; Nagao, T.; Okabe, H.; Matsunaga, K.; Takata, J.; Karube, Y.; Tsuchihashi, R.; Kinjo, J.; Mihashi, K.; Fujioka, T.: Antiproliferative constituents from Umbelliferae plants VII. active triterpenes and rosmarinic acid from Centella asiatica. Biol. Pharmaceut. Bull. 28(1), 173–175 (2005)
Zaidan, M.R.; Noor Rain, A.; Badrul, A.R.; Adlin, A.; Norazah, A.; Zakiah, I.: In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop. Biomed. 22(2), 165–170 (2005)
Indubala, J.; Ng, L.: Herbs: The Green Pharmacy of Malaysia, pp. 76–77. Vinpress Sdn. Bhd., Kuala Lumpur (2000)
Maquart, F.X.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J.P.: Stimulation of collagen synthesis in fibroblast cultures by a triterpene extracted from Centella asiatica. Connect. Tissue Res. 24(2), 107–120 (1990)
Tenni, R.; Zanaboni, G.; De Agostini, M.P.; Rossi, A.; Bendotti, C.; Cetta, G.: Effect of the triterpenoid fraction of Centella asiatica on macromolecules of the connective matrix in human skin fibroblast cultures. Ital. J. Biochem. 37(2), 69–77 (1987)
Mehrban, N.; Smith, A.M.; Grover, L.M.: Evaluation of iota-carrageenan as a potential tissue engineering Scaffold. In: 8th World Congress of Chemical Engineering (2009)
Gan, T.; Zhang, Y.; Guan, Y.: In situ gelation of P (NIPAM-HEMA) microgel dispersion and its applications as injectable 3D cell scaffold. Biomacromolecules 10(6), 1410–1415 (2009)
Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T.: Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Can. Res. 70(22), 9319–9328 (2010)
Weiss, M.S.; Bernabé, B.P.; Shikanov, A.; Bluver, D.A.; Mui, M.D.; Shin, S.; Broadbelt, L.J.; Shea, L.D.: The impact of adhesion peptides within hydrogels on the phenotype and signaling of normal and cancerous mammary epithelial cells. Biomaterials 33(13), 3548–3559 (2012)
Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; Gray, J.W.; Bissell, M.J.: The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 1(1), 84–96 (2007)
Rahmanzadeh, R.; Rai, P.; Celli, J.P.; Rizvi, I.; Baron-Lühr, B.; Gerdes, J.; Hasan, T.: Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer. Can. Res. 70(22), 9234–9242 (2010)
Han, J.; Chang, H.; Giricz, O.; Lee, G.Y.; Baehner, F.L.; Gray, J.W.; Bissell, M.L.; Kenny, P.A.; Parvin, B.: Molecular predictors of 3D morphogenesis by breast cancer cell lines in 3D culture. PLoS Comput. Biol. 6(2), e1000684 (2010)
Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C.: Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31(32), 8494–8506 (2010)
Eke, I.; Cordes, N.: Dual targeting of EGFR and focal adhesion kinase in 3D grown HNSCC cell cultures. Radiother. Oncol. 99(3), 279–286 (2011)
Friedrich, J.; Ebner, R.; Kunz-Schughart, L.A.: Experimental anti-tumor therapy in 3-D: spheroids–old hat or new challenge? Int. J. Radiat. Biol. 83(11–12), 849–871 (2007)
Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J.: Engineering tumors with 3D scaffolds. Nat. Methods 4(10), 855–860 (2007)
Martin, M.D.; Fingleton, B.; Lynch, C.C.; Wells, S.; Mclntyre, J.O.; Piston, D.W.; Matrisian, L.M.: Establishment and quantitative imaging of a 3D lung organotypic model of mammary tumor outgrowth. Clin. Exp. Metastasis 25(8), 877–885 (2008)
Horning, J.L.; Sahoo, S.K.; Vijayaraghavalu, S.; Dimitrijevics, S.; Vasir, J.K.; Jain, T.K.; Panda, A.K.; Labhasetwar, V.: 3-D tumor model for in vitro evaluation of anticancer drugs. Mol. Pharm. 5(5), 849–862 (2008)
Kang, S.W.; Bae, Y.H.: Cryopreservable and tumorigenic three-dimensional tumor culture in porous poly(lactic-co-glycolic acid) microsphere. Biomaterials 30(25), 4227–4232 (2009)
Tasso, R.; Augello, A.; Carida’, M.; Postiglione, F.; Tibiletti, M.G.; Bernasconi, B.; Astigiano, S.; Fais, F.; Truini, M.; Cancedda, R.; Pennesi, G.: Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis 30(1), 150–157 (2009)
da Silva, J.; Lautenschläger, F.; Sivaniah, E.; Guck, J.R.: The cavity-to-cavity migration of leukaemic cells through 3D honey-combed hydrogels with adjustable internal dimension and stiffness. Biomaterials 31(8), 2201–2208 (2010)
Blanco, T.M.; Mantalaris, A.; Bismarck, A.; Panoskaltsis, N.: The development of a three-dimensional scaffold for ex vivo biomimicry of human acute myeloid leukaemia. Biomaterials 31(8), 2243–2251 (2010)
Campbell, J.J.; Davidenko, N.; Caffarel, M.M.; Cameron, R.E.; Watson, C.J.: A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS ONE 6(9), e25661 (2011)
Tan, P.H.; Aung, K.Z.; Toh, S.L.; Goh, J.C.; Nathan, S.S.: Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology. Biomaterials 32(26), 6131–6137 (2011)
Ma, Y.J.; Bryce, N.S.; Whan, R.M.; Xiao, L.; Li, K.; Ruys, A.; Hambley, T.W.; Boughton, P.: Growth of DLD-1 colon cancer cells on Variotis scaffolds of controlled porosity: a preliminary study. J. Biomim. Biomater. Tissue Eng. 8(1), 79–89 (2010)
Zubairi, S.I.: Development of Biomimetic PHB and PHBV Scaffolds for a Three Dimensional (3-D) in vitro Human Leukaemia Model. Imperial College London, London (2013)
Aizad, S.; Yahaya, B.H.; Zubairi, S.I.: Development of a rapid continuous flow salt leaching kit for fabrication of poly (3-hydroxybutyric acid-co-3-hydroxyvalerate) (PHBV) porous 3-D scaffold. Jurnal Teknologi (2015). https://doi.org/10.11113/jt.v75.3903
Almarza, A.J.; Athanasiou, K.A.: Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann. Biomed. Eng. 33(7), 943–950 (2005)
Vunjak-Novakovic, G.; Radisic, M.: Cell seeding of polymer scaffolds. In: Biopolymer Methods in Tissue Engineering, pp. 131–145. Springer, Berlin (2004)
Radisic, M.; Euloth, M.; Yang, L.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G.: High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol. Bioeng. 82(4), 403–414 (2003)
Li, Y.; Ma, T.; Kniss, D.A.; Lasky, L.C.; Yang, S.T.: Effects of filtration seeding on cell density, spatial distribution, and proliferation in nonwoven fibrous matrices. Biotechnol. Prog. 17(5), 935–944 (2001)
Liu, X.; Ma, P.X.: Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 32(3), 477–486 (2004)
Hamilton, D.W.; Riehle, M.O.; Monaghan, W.; Curtis, A.S.G.: Articular chondrocyte passage number: influence on adhesion, migration, cytoskeletal organisation and phenotype in response to nano- and micro-metric topography. Cell Biol. Int. 29(6), 408–421 (2005)
Yang, K.G.A.; Saris, D.B.F.; Geuze, R.E.; van Rijen, M.H.P.; van der Helm, Y.J.M.; Verbout, A.J.; Creemers, L.B.; Dhert, W.J.A.: Altered in vitro chondrogenic properties of chondrocytes harvested from unaffected cartilage in osteoarthritic joints. Osteoarthr. Cartil. 14(6), 561–570 (2006)
Ushida, T.; Furukawa, K.; Toita, K.; Tateishi, T.: Three-dimensional seeding of chondrocytes encapsulated in collagen gel into PLLA scaffolds. Cell Transpl. 11(5), 489–494 (2002)
Li, Z.; Gunn, J.; Chen, M.H.; Cooper, A.; Zhang, M.: On-site alginate gelation for enhanced cell proliferation and uniform distribution in porous scaffolds. J. Biomed. Mater. Res. Part A 86A(2), 552–559 (2008)
Zubairi, S.I.: Porous three dimensional (3-D) scaffolds of poly(3-hydroxybutyric acid) (PHB) and poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) (PHBV): determination of salt leaching efficiency of solvent casting particulate leaching (SCPL) process. Adv. Environ. Biol. 8(10), 925–932 (2014)
Zubairi, S.I.; Bismarck, A.; Mantalaris, A.: The effect of surface heterogeneity on wettability of porous three dimensional (3-D) scaffolds of poly(3-hydroxybutyric acid) (PHB) and poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) (PHBV). Jurnal Teknologi 75(1), 305–312 (2015)
Zubairi, S.I.; Mantalaris, A.; Bismarck, A.: A rapid approach to the pore interconnectivity analysis of porous three dimensional scaffolds of poly(3-hydroxybutyric acid) (PHB) and poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) (PHBV) through non-invasive color staining method. Sains Malays. 44(9), 1531–1356 (2015)
Shen, F.; Zhang, E.; Wei, Z.: Surface bio-modification of poly(hydroxybutyrate-co-hydroxyhexanoate) and its aging effect. Colloids Surf., B 73(2), 302–307 (2009)
Zubairi, S.I.; Bismarck, A.; Koutinas, A.; Panoskaltsis, N.; Mantalaris, A.: Surface treatment of poly(3-hydroxybutyric acid) (PHB) and poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) (PHBV) porous three-dimensional scaffolds with an improved thickness to enhance cell-biomaterial adhesion and interactions. In: 24th European Conference on Biomaterials—Annual Conference of the European Society for Biomaterials (2011).
Chan, S.W.; Mirhosseini, H.; Taip, F.S.; Ling, T.C.; Tan, C.P.: Comparative study on the physicochemical properties of κ-carrageenan extracted from Kappaphycus alvarezii (doty) doty ex Silva in Tawau, Sabah, Malaysia and commercial κ-carrageenans. Food Hydrocoll. 30(2), 581–588 (2013)
Aizad, S.; Yahaya, B.H.; Zubairi, S.I.: Carboxy-methyl-cellulose (CMC) hydrogel-filled 3-D scaffold: preliminary study through a 3-D antiproliferative activity of Centella asiatica extract. AIP Conf. Proc. 1678, 050005 (2015)
International A: Standard Test Method for In Vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants. ASTM International, West Conshohocken (2004)
Aizad, S.; Khairi, N.M.; Yahya, B.H.; Zubairi, S.I.: A novel anti-proliferative activity (EC50) of pegaga (Centella asiatica) extract through in vitro 3-D culture microenvironment. Jurnal Teknologi 79(2), 1–10 (2017)
Chen, G.Q.; Wu, Q.: The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26(33), 6565–6578 (2005)
Zubairi, S.I.; Suradi, H.; Mutalib, S.A.A.; Othman, Z.S.; Bustaman, N.; Wan Musa, W.R.M.: Preliminiry study on kinetic solid-liquid extraction and bio-active components analysis of Hibiscus rosa-sinensis leaves. Malays. J. Anal. Sci. 18(1), 43–57 (2014)
Abidin, N.Z.; Janam, A.; Zubairi, S.I.: Adsorption of saponin compound in Carica papaya leaves extract using weakly basic ion exchanger resin. AIP Conf. Proc. 1784, 030039 (2016)
Mohd Fazil, F.N.; Mohd Azzimi, N.S.; Yahaya, B.H.; Kamalaldin, N.A.; Zubairi, S.I.: Kinetics extraction modelling and antiproliferative activity of Clinacanthus nutanswater extract. Sci. World J. (2016). https://doi.org/10.1155/2016/7370536
Arullappan, S.; Rajamanickam, P.; Thevar, N.; Kodimani, C.C.: In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop. J. Pharmaceut. Res. 13(9), 1455–1461 (2014)
Patel, S.; Gheewala, N.; Suthar, A.; Shah, A.: In-vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int. J. Pharm. Pharmaceit. Sci. 1(1), 38–46 (2009)
Schuldiner, M.; Eiges, R.; Eden, A.; Yanuka, O.; Itskovitz-Eldor, J.; Goldstein, R.S.; Benvenisty, N.: Induced neuronal differentiation of human embryonic stem cells. Brain Res. 913(2), 201–205 (2001)
Chen, C.; Okayama, H.: High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7(8), 2745–2752 (1987)
Ceccarini, C.; Eagle, H.: pH as a determinant of cellular growth and contact inhibition. Proc. Natl. Acad. Sci. 68(1), 229–233 (1971)
Reeves, R.; Ribeiro, A.; Lombardo, L.; Boyer, R.; Leach, J.B.: Synthesis and characterization of carboxymethylcellulose-methacrylate hydrogel cell scaffolds. Polymers 2(3), 252–264 (2010)
Kuebler, W.M.; Parthasarathi, K.; Lindert, J.; Bhattacharya, J.: Real-time lung microscopy. J. Appl. Physiol. 102(3), 1255–1264 (2007)
Hoang, A.T.N.; Chen, P.; Juarez, J.; Sachamitr, P.; Billing, B.; Bosnjak, L.; Dahlén, B.; Coles, M.; Svensson, M.: Dendritic cell functional properties in a three-dimensional tissue model of human lung mucosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 302(2), L226–L237 (2012)
Barrila, J.; Radtke, A.L.; Crabbé, A.; Sarker, S.F.; Herbst-Kralovetz, M.M.; Ott, C.M.; Nickerson, C.A.: Organotypic 3D cell culture models: using the rotating wall vessel to study host–pathogen interactions. Nat. Rev. Microbiol. 8(11), 791–801 (2010)
Basile, A.; Ferrara, L.; Del Pezzo, M.; Mele, G.; Sorbo, S.; Bassi, P.; Montesano, D.: Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana, Mart. J. Ethnopharmacol. 102(1), 32–36 (2005)
Pittella, F.; Dutra, R.; Junior, D.; Lopes, M.T.; Barbosa, N.: Antioxidant and cytotoxic activities of Centella asiatica (L.) Urb. Int. J. Mol. Sci. 10(9), 3713–3721 (2009)
Ruszymah, B.H.I.; Chowdhury, S.R.; Manan, N.A.B.A.; Fong, O.S.; Adenan, M.I.; Saim, A.B.: Aqueous extract of Centella asiatica promotes corneal epithelium wound healing in vitro. J. Ethnopharmacol. 140(2), 333–338 (2012)
Abidin, N.Z.; Zubairi, S.I.; Omar, H.; Empungan, A.K.; Kamalaldin, N.A.; Yahaya, B.H.: Proliferative activity of saponin-free Carica papaya extract on human lung fibroblast cell (IMR90). Jurnal Teknologi (Sci. Eng.) 78(11–3), 41–49 (2016)
Zubairi, S.I.; Mantalaris, A.; Bismarck, A.; Aizad, S.: Polyhydroxyalkanoates (PHAs) for tissue engineering applications: biotransformation of palm oil mill effluent (POME) to value-added polymers. Jurnal Teknologi (Sci. Eng.) 78(1), 13–29 (2016)
Che Johari, N.S.; Aizad, S.; Zubairi, S.I.: Efficacy study of carrageenan as an alternative infused material (filler) in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) porous 3-D scaffold. Int. J. Polym. Sci. (2017). https://doi.org/10.1155/2017/5029194
Liuyun, J.; Yubao, L.; Chengdong, X.: Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J. Biomed. Sci. 16, 65 (2009)
Kamalaldin, N.A.; Jaafar, M.; Zubairi, S.I.; Yahaya, B.H.: Physico-mechanical properties of HA/TCP pellets and their three-dimensional (3-D) biological evaluation in vitro. In: Advances in Experimental Medicine and Biology—Innovations in Cancer Research and Regemerative Medicine, pp. 1–15 (2017).
Brooks, A.N.; Turkarslan, S.; Beer, K.D.; Lo, Y.F.; Baliga, N.S.: Adaptation of cells to new environments. Wiley Interdiscipl. Rev. Syst. Biol. Med. 3(5), 544–561 (2011)
Cukierman, E.; Pankov, R.; Yamada, K.M.: Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 14(5), 633–640 (2002)
Acknowledgements
The authors would like to thank the Ministry of Science, Technology and Innovation (MOSTI), Ministry of Higher Education (MOE) Malaysia and Universiti Kebangsaan Malaysia (UKM) for providing financial support to this project (06-01-02-SF1271, FRGS/2/2013/TK04/UKM/03/1, GGPM-2013-078, GUP-2018-057 and GUP-2018- 080).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aizad, S., Zubairi, S.I., Yahaya, B.H. et al. Centella asiatica Extract Potentiates Anticancer Activity in an Improved 3-D PHBV-Composite-CMC A549 Lung Cancer Microenvironment Scaffold. Arab J Sci Eng 46, 5313–5325 (2021). https://doi.org/10.1007/s13369-020-05072-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05072-7