Abstract
Where allochthonous large mammals, such as the wild boars, occur in high density, human-wildlife conflicts may arise. Thus, assessing their spatio-temporal patterns is paramount to their management. We studied the wild boars on Elba island, Italy, where they have been introduced and are perceived as pests to address their occurrence and impact of foraging on natural habitat. We surveyed the western island with three camera trapping surveys within one year. We found that the species' estimated occupancy probability was higher in summer-autumn (0.75 ± 0.14) and winter-early spring (0.70 ± 0.10) than in spring–summer (0.53 ± 0.15), whereas detection probability did not vary. Occupancy was significantly associated with lower elevation and woodland cover. Lower site use of wild boars during spring–summer might reflect lower food availability in this season and/or boars’ movements towards landfarms outside the sampled area. Detectability increased with proximity to roads during spring–summer and decreased with humans’ relative abundance in other periods. Boars were mainly nocturnal, with decreasing overlap with human activity when human presence was higher in the park. Soil degradation caused by wild boars was higher in pine plantations, which is the cover with a lower conservation interest. The spatio-temporal activity of wild boars on the island appears driven by seasonal preferences for food-rich cover and avoidance of human disturbance. The lowered site use in months with lower resources could partially reflect increased proximity to settled and farmed areas, which may trigger crop-raiding and the negative perception by residents.
Similar content being viewed by others
Introduction
The wild boar (Sus scrofa) is an ungulate that often triggers a wide range of human-wildlife conflicts, whose demographic history in Europe is complex and affected by various reintroductions and translocations. The species is native to the Eurasian continent (Barrios-Garcia and Ballari 2012), with two native forms in central Italy (Apollonio et al. 1988; Iacolina et al. 2016), and it carries out crucial ecological functions (Selva et al. 2005; Fonseca 2008; Mori et al. 2017). Yet it is often associated to a broad range of socio-economic issues primarily related to its high abundance (Bosch et al. 2016; Aguillar et al. 2018; Jägerbrand and Green 2018), the ability to colonise novel environments, including suburban and urban areas, and its impacts on croplands and harvests (Herrero et al. 2006; Schley et al. 2008). Its reputation as problematic wildlife is often exacerbated where it is non-native, its natural predators are absent, or wildlife management is not properly conducted (Bieber and Ruf, 2005; Toïgo et al, 2008). In the last 30 years, the distribution range of the wild boar has largely expanded due to anthropogenic and environmental factors (Bieber and Ruf, 2005; Geisser and Reyer, 2005; Hearn et al. 2014; Massei et al. 2014), with the uncontrolled restocking for hunting purposes being one of the major causes. This practice had led to the introduction of such highly plastic and prolific species on islands, including the Tuscan Archipelago before it became a national park in 1996 (Meriggi et al. 2015). Island ecosystems are particularly vulnerable to the effects of introduced populations for the geographic isolation and the higher specialisation of native species (Russell et al. 2017). In this context, wild boars can reach high densities since natural predators and competitors are usually absent. As an important ecological engineer (Jones et al. 1994), boars can trigger knock-off effects on biocenosis that span from the extensive rooting of slopes and soils, ground aeration, uprooting and trampling of seedlings, the creation of germination niches for plants, and the direct consumption of flora and fauna with potential high conservation interest (Massei and Genov 2004; Sendom and Hughes 2012).
In the Tuscan Archipelago National Park (TANP), wild boars have been introduced and occur only on Elba where they have been recorded for the first time at the beginning of the twentieth century (Damiani 1923). Subsequently, other individuals from eastern Europe were introduced in the 1960s as a game species (Meriggi et al. 2015). The absence of natural predators and direct competitors on the island allowed the new population to increase and expand over the whole area, taking advantage of its generalist diet and high fecundity. In particular, the western part of the island, designated as a national park with prohibited hunting, is assumed to host a relatively higher wild boars’ presence, potentially impacting natural habitats and agricultural fields (Monaco et al. 2010). Elba is also a popular tourist destination, and the presence of boars often raises concerns for human safety, especially in summer when incursions towards farmlands and residential areas are documented (Giannini and Montauti 2010). Their impacts on the island include collision with vehicles, destruction of dry walls, crop damages, degradation of meadows and traditional agricultural systems as well as native flora and fauna in general (Serra et al. 2001; Giannini and Montauti 2010; Acosta and Ercole 2015; Meriggi et al. 2015). Its feeding behaviour, characterised by the typical rooting activity, can alter and erode the soil substrate by removing the superficial vegetation stratum (Siemann et al. 2009; Wirthner et al. 2012). At present, contrasting information is available concerning the effect of the wild boars’ feeding behaviour, although previous studies have demonstrated that their rooting activity causes a decline of native flora and support plant invasions, especially on islands where ungulates were not historically present (Aplet et al. 1991; Oldfield and Evans 2016). In a few decades, wild boars became so widespread and the socio-economic impacts became so severe that since 1997 the TANP has promoted a series of management actions with an average of 600 individuals captured each year and approximately 12,000 wild boars removed from the park (TANP 2018). However, while the economic damage caused by wild boars has been documented for this island (Meriggi et al. 2015), no studies have assessed the spatial and temporal patterns of wild boars’ occurrence, nor the impact of foraging on natural habitat.
Here, we studied wild boars in the western part of Elba using camera trapping during three seasons, and we also sampled the status of soil and vegetation. We aimed to (1) assess wild boar spatial distribution and habitat association in relation to environmental and anthropogenic variables, as well as variations across three sampling seasons with different trophic resources and food availability; (2) determine the temporal activity and variation among seasons of wild boars in relation to human presence; and (3) assess the impact of wild boar foraging on soil and ground vegetation by quantifying the intensity of the rooting activity across macrohabitats.
Methods
Study area
The study was conducted on the western part of Elba island (42° 46′ 20.4′′ N, 10° 10′ 14.4′′ E), and within the borders of the TANP, in Central Italy (Fig. 1). The island extends for 302 km2, while the park’s area encompasses 206.3 km2 (Meriggi et al. 2015). Elba is characterised by a Mediterranean climate, with a yearly mean temperature of 16.5 °C, dry summers and mild winters, and a localised colder microclimate with sporadic snowfalls on the top of the Mount Capanne, which represents the highest peak with 1016 m a.s.l. (Foggi et al. 2006). Mean yearly precipitations amount to 595 mm, with periods of drought during the summer months, characterised by scanty rainfalls (down to 13 mm), and temperatures exceeding 30 °C during the hottest time of the day (Meriggi et al. 2015). The study area is characterised by woodlands mainly located on the northern slopes, and several types of maquis and garrigues; these last two results to be the most represented habitats on the southern slopes. Patches of pine plantations (Pinus sp.) are also scattered along the mountain slopes and derived from the reforestation policies of the 1950s. Thus, we distinguished five major macrohabitats: holm-oak woods (Quercus ilex), chestnut groves (Castanea sativa), pine plantations, low Mediterranean maquis, including garrigues, characterised by rosemary (Rosmarinus officinalis), lavender (Lavandula stoechas) and rockroses (Cistus sp. pl.) (hereafter “low maquis”), and Mediterranean maquis with vegetation > 1 m characterised by strawberry trees (Arbutus unedo) and tree heath (Erica arborea) (hereafter “tall maquis”). Urban and agricultural areas are located just outside the borders of the TANP, with fields mainly cultivated as orchards and vineyards, and a major paved road connecting the towns that rings the edge of the park.
Data collection
Boars’ detections were collected using camera traps (CTs) deployed in the study area (from 160 to 1000 m a.s.l.) between April 2018 and April 2019 (Fig. 1). The survey consisted of three separate sampling periods, each deploying 80 camera stations: from the 27th of April to the 15th of July 2018 (spring–summer), from the 1st of September to the 18th of November 2018 (late summer-autumn) and from the 18th of January to the 8 of April 2019 (winter-early spring). For each sampling period, cameras were active in the field for a minimum of 19 days and, due to equipment and time constraints, we used 20 motion-triggered camera traps of three different brands (Ltl Acorn – Shenzhen, Guangdong, China; Spromise – Shenzhen, Guangdong, China; and U-way – Atlanta, Georgia, USA) deployed in four consecutive arrays of 20 CTs each. The devices had similar technical characteristics as they mounted IR flash and 0.8–1 s trigger speed. Due to the dense vegetation and the harsh terrain, CT stations were placed in proximity of trekking trails, about 20 m off-trails, following the altitudinal gradient of the mountains, with approximately 500 m spacing between cameras. Every camera trap was secured to trees’ trunks at approximately 50 cm from the ground, and in proximity of signs of wildlife presence (scats, footprint, etc.). We did not use baits or lure. Eight CTs were moved after the first sampling period due to the inaccessibility of the terrain, whereas between sampling periods, cameras were placed in a buffer of approximately 20 m around the selected CT station point yet trying to be as close as possible to the original sampling location. We collected environmental data (i.e. macrohabitat type, elevation, dominant vegetation type and percentage of tree, shrub and grass) at each CT station to be used as covariates in the occupancy analyses (details below).
To assess the impact of boar foraging on soil and ground vegetation, we followed the protocol in Lazzaro et al. (2015). Thus, we deployed 80 plots of 10 × 2 m centred on the CTs. The vegetation survey was carried out from the 29th of April to the 5th of May 2019, and plots were distributed across all five macrohabitats. We estimated the percentage of torn-off ground within the plot, as a proxy of soil degradation and intensity of wild boar rooting activity, and inspected ground quality within each plot by using a discrete scale from “1” (i.e. well-preserved ground with high plant species richness) to “3” (i.e. highly degraded soil with highly-damaged vegetation, signs of erosion and/or soil compactness) (see Suppl. Table 1 for variables specification and description).
Covariates for occupancy modelling
To estimate wild boars’ spatial occurrence across the study area, we modelled occupancy and detection probability (details below) using ten environmental and anthropogenic variables (see Suppl. Table 1). Collinearity among them was inspected using a correlation coefficient with r = 0.5 as threshold. Covariates were: (1) the camera trap model, since trigger speed can affect the probability to record the target species (Rovero et al. 2013); (2) the Relative Abundance Index of human activity (i.e. RAI human), estimated at CT station-level with a 1-day interval, since human presence can affect the activity patterns of large mammalian species (Oberosler et al. 2017) in terms of both detection and occupancy; (3) the distance to the closest road, measured with the built-in tool in Quantum Gis (QGis Development Team 2019) over a 1:10,000 scale map. We considered this variable a proxy of anthropogenic disturbance since proximity to roads can influence wildlife behaviour (Cooke et al. 2019), potentially affecting both occupancy and detection; (4) the macrohabitat type in terms of dominant species (i.e. low maquis, tall maquis, pine plantation, chestnut groves, and holm-oak wood) and the percentage of bush cover since both detection and occupancy probability can be affected by habitat characteristics (Gu and Swihart 2002). Additionally, occupancy was also modelled using: (5) the elevation, considered a proxy of varying trophic resources and habitat characteristics; (6) the slope aspect of the mountain (north–south), since the different intensity in solar radiation can determine optimal microhabitat characteristics (Maren et al.2015); (7) the percentage of grass and tree cover, and (8) the dominant vegetation type (i.e. wood, understory, shrub), together depicting the habitat structure. We also measured the distance of every camera station to the closest main town, but then excluded it from the analyses since it resulted correlated to the distance to the closest main road. Other covariates were not collinear. In summary, we predicted detectability to increase with faster camera trap, decrease with proximity to roads and higher RAI of humans, and to be affected by habitat features; we predicted occupancy to be affected by environmental characteristics associated to resource acquisition and refugia.
Data analyses
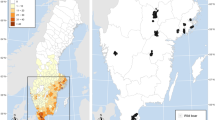
Camera trap images were annotated using the open-source software Wild.ID (Fegraus and MacCarthy 2016), which allowed for species classification using the IUCN taxonomy. From the resultant file, we extracted all records related to the wild boar and estimated for each separate season the number of independent events (with a 30 min interval between photographs) to avoid counting multiple times the same individual at the same CT station. With the independent events, we estimated the RAI for each sampling period calculated as events on sampling effort and multiplied by 100. We also derived the naïve occupancy, i.e. the proportion of sites occupied on sites sampled. With the site- and season-specific RAI values, we created a proportional symbol map in the open-source software QGis to display seasonal patterns of wild boars’ raw detections and used the wild boar RAI as a proxy for the intensity use of every CT station (Sollmann 2018).
Spatial variations: occupancy modelling
To estimate the wild boar “true” occupancy (Ψ) across the study area (i.e. with account for imperfect detection p), we used single-species occupancy models (MacKenzie et al. 2002), in R (R Core Team 2019) implemented in “unmarked” (Fiske and Chandler 2011). We decided to use single-season models instead of dynamic models as our aim was to determine habitat association in each "season" rather than evaluating dynamic parameters. In addition, as described in “Data collection”, we could not ensure complete consistency in sites samples across seasons. We arranged detection/non-detection data as matrices of sites by surveys (i.e. sampling occasion), with a resolution of 1 day. Each entry indicated if the species was observed (1) at site i on survey j or not (0). A site i that was not sampled on survey j was scored as NA. We then used these matrices as the input for the single-season occupancy models. We standardised covariates to have mean 0 and a unit standard deviation. In addition to the null model (that assumes constant Ψ and p), we built several models using different combinations of anthropogenic and environmental covariates on both Ψ and p based on the aforementioned ecological assumptions. Specifically, we first assessed the best-supported variables that can account for imperfect detection by testing various models with different variables combination on p, and then used the first-ranked model for the detection to determine the best model/s for Ψ. For both steps of the model selection, we assessed model fit by ranking the candidate models with the Akaike Information Criterion (AIC). For the final models, we considered as statistically best-supported those with Δ AIC < 2. In case of multiple top-ranked models we used the package “AICcmodavg” (Mazzerolle 2019) to average them and derive predictions for Ψ and p.
Temporal variation: diel activity patterns
To investigate the temporal pattern of wild boars’ occurrence, we used a non-parametric Kernel Density Estimation (KDE) function, using the package “Overlap” (Meredith and Ridout 2014), following the protocol in Ridout and Linkie (2009). For each sampling period, we used the timestamp of each independent event, derived with a 30 min interval to create an activity distribution curve. In addition, to assess seasonal differences in boars’ activities to the disturbance in the park (i.e. human presence), we estimated the seasonal overlap coefficient Δ, ranging from 0 (no overlap) to 1 (complete overlap), between the wild boars and humans by performing pairwise comparisons of their diel activity patterns. We then generated distribution overlap values by performing 999 bootstraps to estimate confidence intervals (Ridout and Linkie 2009; Meredith and Ridout 2014). We expected the overlap to be smaller with greater disturbance.
Vegetation analysis
To evaluate the spatial pattern of rooting activity, thus the impact of wild boar’s foraging on soil and ground vegetation across macrohabitats, we used the percentage of overturned ground as a proxy of soil degradation and intensity of rooting activity in the five macrohabitat types. Hence, we implemented a Binomial Generalised Linear Model (GLM) with the percentage of overturned ground as the response and the macrohabitat types (with five levels) as the explanatory variable, under the expectation that the amount of overturned soil would be higher in food-rich macrohabitats. The analysis was carried out using the package “stats”. Model assumptions were inspected following Zuur et al. (2009). Additionally, we used a Kendall correlation coefficient to inspect potential correlation between overturned soil and ground degradation.
Results
During the surveys, nine cameras produced no data as they were either stolen or malfunctioning, yet we reached a robust sampling effort in every season (> 1000 days, Table 1). We detected the presence of four medium-to-large wild mammal species, three domestic species, several small mammals and birds, and various human detections in the forms of trekkers/bikers and vehicles (see Suppl. Table 2). Based on raw detection indices, wild boars appeared among the most photographed wildlife on the island, with their raw detections and activity varying greatly across seasons (Table 2, Fig. 2).
Proportional symbol map representing the spatial activity pattern of the wild boars in the western part of Elba island, Italy, during three separate sampling seasons from April 2018 to April 2019. Circles represent the site-specific Relative Abundance Index (RAI) with size varying according to value intervals, while colours represent the different sampling seasons
For every season, the “null” model (i.e. no covariates) was the least supported. For each sampling period, multiple models resulted best-supported (Δ AIC < 2); hence we estimated Ψ and p by averaging them (Table 3, see also Suppl. Table 3). Models results showed that the wild boars had different spatial occurrence levels in the study area across seasons, with the spring–summer period displaying the lowest occupancy probability (Ψ = 0.53 ± 0.15 SE) compared to late summer-autumn (Ψ = 0.75 ± 0.14) and winter-early spring (Ψ = 0.70 ± 0.10). Conversely, average detectability was similar across seasons (p1 = 0.19 ± 0.05; p2 = 0.16 ± 0.03; p3 = 0.12 ± 0.03). Habitat characteristics associated with the wild boar’s Ψ and p varied slightly according to the sampling season (Table 4). Elevation and vegetation types were the covariates significantly associated with its occupancy probability (Figs. 3, 4). In particular, the wild boar occupancy significantly decreased with increasing elevation during spring–summer (− 1.06 ± 0.46, P < 0.05) and winter-early spring (− 1.08 ± 0.45, P < 0.01), while occupancy increased significantly with woodland as main vegetation type for both spring–summer (3.33 ± 0.42, P < 0.05) and late summer-autumn (2.67 ± 0.91, P < 0.01). During this latter period, also the understory (tall maquis) had a significant positive association with the occupancy probability (2.28 ± 1.01, P < 0.05). Only in spring–summer, the low Mediterranean maquis was negatively associated with Ψ (− 2.89 ± 1.57, P = 0.07), while a higher percentage of shrub coverage was positively associated to a higher occupancy probability (1.21 ± 0.63, P = 0.05), although both effects were only marginally significant. The distance to the closest road, the percentage of shrub cover, the habitat types, the human RAI, and camera models were the covariates affecting the wild boar detection probability, though with a seasonal variation. During spring–summer, wild boar detection probability increased significantly in proximity to the main road (0.67 ± 0.16, P < 0.001), whereas it was significantly lower with higher shrub coverage (− 0.53 ± 0.18, P < 0.01). The detection probability had a significant negative association with greater human activity in the study area (RAI human) during both summer-autumn (− 0.36 ± 0.10, P < 0.001) and winter-spring (− 0.50 ± 0.17, P < 0.01). A similar pattern was found also for both the tall Mediterranean maquis (− 0.82 ± 23, P < 0.001 for the second sampling season and − 0.80 ± 0.32, P < 0.01 for the third one) and the low maquis (− 1.95 ± 0.72, P < 0.01 for the second season and − 1.28 ± 0.37, P < 0.001 for the third one) (Table 3). Detection probability was also affected by camera models with U-way trail camera (1.79 ± 0.62, P < 0.01 during spring–summer) and Spromise (0.69 ± 0.25, P < 0.01 during summer-autumn) determining higher detectability.
Estimated occupancy probability (Ψ) of the wild boar (Sus scrofa) on Elba island, Italy. Occupancy was predicted in relation to the three vegetation types (shrub, understory and wood), during late summer-autumn and winter-early spring, that is when this covariate was statistically supported (Δ AIC < 2) and to be included in the average model
The diel activity pattern of wild boars appeared consistent across sampling periods, with the intensity of the activity decreasing after sunrise and increasing during sunset hours (Fig. 5a). On the other hand, the overlap between the wild boars and humans activity patterns appeared smaller during late summer-autumn (Δ = 0.29; 0.23–0.36) which was the period with the most intense human activity, compared to spring–summer (Δ = 0.37; 0.25–0.47) and winter-early spring (Δ = 0.32; 0.18–0.45) (Fig. 5b).
Temporal pattern of wild boars (Sus scrofa) in the western part of Elba island, Italy, from April 2018 to April 2019. On the left, yearly activity pattern with independent events (< 30 min) divided into time slots (0–23) and numbers on the x-axis representing total independent events detected during the same hour (a). On the right, seasonal Kernel density distributions of wild boars and humans and overlaps in their diel activity patterns during each sampled season (b). Figure shows overlap coefficient (Δ) and upper-lower limits for each season
We found a significantly higher percentage of overturned soil, corresponding to a higher intensity of rooting activity, within the pine plantation patches (P < 0.04), and a lower rooting intensity in the low maquis (P = 0.06, Table 5, Fig. 6). Additionally, higher percentage of torn-off ground was positively correlated with higher ground degradation with lower vegetation species richness and highly degraded plants (R = 0.60, P < 0.001).
Discussion
We studied the spatio-temporal activity of wild boars on Elba island and found that the species is widespread across the study area, with an estimated occupancy that seasonally reaches average values of 0.75. Variations in spatial occurrence and diel activity pattern among seasons appear driven by seasonal preferences for food-rich cover and avoidance of human disturbance. In particular, the lowered site use in months with lower resources could partially reflect increased proximity to settled and farmed areas, which may, in turn, trigger crop-raiding and hence the negative perception by residents.
Seasonal movement patterns associated with the availability of food resources are compatible with the "food exploitation hypothesis" proposed by Larter and Gates (1994), with animals adjusting their distribution range to optimise the use of trophic resources in the area. Wild boars exhibit strong responses toward food pulse (Cutini et al. 2013), hence their foraging activity can affect their home ranges, with the use of different areas in different seasons. Our findings, in particular, are consistent with Meriggi et al. (2015), that reports increased damage to orchards and meadows caused by wild boars on Elba island during summer. Moreover, lower abundance of food resources in summer, associated with low precipitation and droughts, has been reported within the park area (Gianniani and Montauti 2010). Indeed we recorded an higher intensity of habitat use, as proxied by RAI values, in the southeastern part of the study area, where small agricultural parcels are present.
The relatively higher occurrence of wild boars within the park that peaks during both the late summer-autumn and winter-early spring suggest a firm association with woodland cover. Several studies have shown that woodlands represent the optimal habitat for wild boars across the year (e.g. Abaigar et al. 1994; Rodrigues et al. 2016; Keuling and Leus 2019), as associated to food provisioning (e.g. chestnuts, acorns, mushrooms, tubers, and wild asparagus), humid and cool microclimate, shadowy coverage from heat and presence of streams and pools. We also found that the Mediterranean low maquis was the least preferred cover, especially during late spring–summer. In fact, this latter macrohabitat mainly develops on the southern slope, and at a higher elevation of the Mount Capanne; it is a very dry and exposed environment dominated by the poisonous Calicotome spinosa and offers limited resources for wild boars. That occupancy of wild boars generally decreased with elevation suggests a preference for lower elevation zones, except in late summer-autumn. This both appears consistent with the presence of the low Mediterranean maquis at a higher elevation and supports Meriggi et al. (2015) findings that damage to crops was higher between 100 and 300 m a.s.l.. In contrast, during late summer-autumn, wild boars’ occurrence was not related to elevation, indicating a stronger association with woodlands along the mountain slopes, potentially driven by fruiting chestnut groves occurring between 600 and 800 m a.s.l..
As predicted, wild boars' detection probabilities were negatively influenced by the anthropogenic disturbance in late summer-autumn and winter-early spring, translating into a marked elusive behaviour when relative human abundance peaked in the park. Similar trends have been reported for other medium-to-large mammals in alpine contexts (Oberosler et al. 2017), confirming the pivotal role of anthropogenic disturbance in detection probability. However, we also found that wild boars can adjust their elusiveness and tolerance to human disturbance when trophic resources are scant since, contrary to our expectation, boars’ detection probability increased in late spring–summer with decreasing distance to the main road. This latter rings the border of the national park, where boars’ detection can be easier at its edges and connects adjacent towns and agricultural fields. Thus, in a context of food scarcity, the ungulate can adopt a bolder behaviour to sources of disturbance, while tendentially avoid human interaction and encounters in periods of high trophic abundance.
The wild boars' nocturnal and crepuscular activity pattern is consistent with the literature from a range of areas (Lemel et al. 2003; Keuling et al. 2008). Moreover, Podgórski Bas et al. (2012) highlighted the behavioural plasticity of this species, with an ability to shift its activity from diurnal to almost exclusively nocturnal in response to different levels of human disturbance. Thus, our findings might reflect increased boars’ elusiveness in areas with higher chances of human encounters. Elba island is a human-dominated landscape, with towns surrounding the borders of the park and many recreational activities within it across seasons, with a peak in late summer-autumn, when we detected the highest human activity (Suppl. Table 2) and the overlap coefficient between human and wild board had the lowest value. Further support to such pattern of human avoidance is given by the map of the intensity in the habitat use, which highlighted that no raw detections were recorded near the most used trekking trails. Besides, the nocturnal/crepuscular behaviour can also ensure access to food resources provided by agricultural fields (Keuling et al. 2008; Podgórski Bas et al. 2012), at times when human control is low.
The evident soil degradation resulting from a greater intensity in the rooting activity found within pine plantations confirms that within the wooded habitat pine plantations are one of the most frequently used by wild boars (Abaigar et al. 1994, Rodrigues et al. 2016). Pinewoods are likely associated with optimal food availability; in particular, a higher abundance of cicada larvae is present in pine plantations, which represent an important food source for wild boars (Genov and Ahmed 2014). This can explain the greater percentage of the overturned ground caused by their foraging strategy (Massei and Genov 1995). However, as a previous study in a Mediterranean ecosystem has shown (Torres-Porras et al. 2015), the greater damage in pinewoods does not necessarily coincide with a higher boar occurrence compared to the other wood forest types, but may be due to an intenser rooting activity given the presence of an underground food source.
Conclusions and management recommendations
Variations in the spatio-temporal activity of wild boars on western Elba island appear driven by the availability of trophic resources, as proxied by habitat cover, and avoidance of anthropogenic disturbance. We provided evidence that these patterns are compatible with perceived conflicts due to crop-raiding by boars and proximity to farmland and urban areas which are elevated in the summer months when food resources in the park are limited. In this scenario, protecting agricultural fields and orchards located close to the park’s borders with electric fences could mitigate the impact caused by wild boars during summer, given the high success rate in keeping wild boars out as reported in the literature (Monaco et al. 2010; Massei et al. 2011). Additionally, electric fencing should be used in conjunction with the management policies currently in force within the National Park. Other forms of mitigation technique, such as dissuasive feeding during the periods of low food availability, could present important drawbacks such as the increase of wild boar reproductive rate (Monaco et al. 2010). The high density of wild boars’ in the park appeared to impact the soil and vegetation, although this may determine substantial damage only in the pine plantations, which are of low conservation interest as they do not represent an autochthonous habitat on the island (Gatteschi and Arretini 1989; Maestre and Cortina 2004). However, despite soil damage that seemingly occur more in pine patches, also the other woodland types suffer from wild boars’ rooting behaviour, and we acknowledge that further research is required to better understand the magnitude of wild boars’ ecological effect on soil properties and plant species diversity.
References
Abaigar T, del Barrio G, Vericad JR (1994) Habitat preferences of wild boar (Sus scrofa L., 1758) in a Mediterranean environment. Indirect evaluation by signs. Mammalia 58(2):201–210
Acosta ATR, Ercole S (2015) Gli habitat delle coste sabbiose italiane: ecologia e problematiche di conservazione (Eds). ISPRA, Technical report, 215/2015. (In Italian)
Aguillar XF, Gottschalk M, Aragon V et al (2018) Urban wild boars and risk of zoonotic streptococcus suis. Spain Emerg Infect Dis 24(6):1083–1086
Aplet G, Anderson S, Stone C (1991) Association between feral pig disturbance and the composition of some alien plant assemblages in Hawaii Volcanoes National Park. Vegetation 95:55–62
Apollonio M, Randi E, Toso S (1988) The systematics of the wild boar (L.) in Italy. Ital J Zool 55(1–4):213–221
Barrio-Garcia MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions 14:2283–2300
Bieber C, Ruf T (2005) Population dynamics in Wild Boar Sus scrofa: ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. J Appl Ecol 42(6):1203–1212
Bosch J, Rodríguez A, Iglesias I, Muñoz MJ, Jurado C, Sánchez-Vizcaíno JM, de la Torre A (2016) Update on the risk of introduction of African Swine Fever by wild boar into disease-free European Union Countries. Transbound Emerg Dis 64(5):1424–1432
Cooke SC, Balmford A, Johnston A, Massimino D, Newson SE, Donald PF (2019) Road exposure and the detectability of birds in field survey. Ibis 162(3):885–901
Cutini A, Chianucci F, Chirichella R, Donaggio E, Mattioli L, Apollonio M (2013) Mast seeding in indigenous forest of the northern Apennines (Italy) and its influence on wild boar population dynamics. Ann For Sci 70(5):493–502
Damiani G (1923) La Fauna (Eds). In: L’Elba I, Foresi S, pp 103–129. (In Italian)
Fiske I, Chandler R (2011) Unmarked: an R package for fitting hierarchical models of occurrence and abundance. J Stat Softw 43:1–23
Fegraus E, MacCharty J (2016) Camera trap data management and interoperability. In: Rovero F, Zimmermann F (eds) Camera trapping for wildlife research. Pelagic Publisher, Exeter
Foggi B, Cartei L, Pignotti L, Signorini MA, Viciani D, Dell’Olmo L, Menicagli E (2006) Il paesaggio vegetale dell’isola D’Elba (Arcipelago Toscano). Studio fitosociologico e cartografico Fitosociologia 43(1):3–95
Fonseca C (2008) Winter habitat selection by wild boar Sus scrofa in southeastern Poland. Eur J Wildl Res 54(2):361
Gatteschi P, Arretini C (1989) Indagine sui rimboscamenti dell’Arcipelago Toscano. Dipartimento Agricoltura Foreste Regione Toscana, Firenze. (In Italian)
Genov PV, Ahmed A (2014) Cicada orni L. in the food of wild boar in the Regional Park Maremma—Toscana. Italy Ecol Balk 5:71–73
Geisser H, Reyer HU (2005) The influence of food and temperature on population density of wild boar Sus scrofa in the Thurgau (Switzerland). J Zool 267(1):89–96
Giannini, F. and Montauti, G. 2010. Controllo del cinghiale e conflitti sociali: il caso del Parco Nazionale Arcipelago Toscano. In: Monaco A, Carnevali L, Toso S (eds). Linee guida per la gestione del cinghiale (Sus scrofa) nelle aree protette. 2° edizione. Quad. Cons. Natura, 34, Min. Ambiente – ISPRA. Technical report, 86 – 87. (In Italian)
Gu W, Swihart RK (2002) Absent or undetected? Effects of non-detections of species occurrence on wildlife-habitat model. Biol Converv 116:195–203
Hearn R, Watkins C, Balzaretti R (2014) The cultural land use implications of the reappearance of the wild boar in North West Italy: a case study of the Val di Vara. J Rural Stud 36:52–36
Herrero J, García-Serrano A, Cuoto S, Otuño VM, García-González R (2006) Diet of wild boar (Sus scrofa L.) and crop damage in an intensive agroecosystem. Eur J Wildl Res 52:245–250
Iacolina L, Scandura M, Goedbloed DJ, Alexandri P, Crooijmans RPMA et al (2016) Genomic diversity and differentiation of a managed island wild boar population. Heredity 116(1):60–67
Jägerbrand AK, Green IM (2018) Consequences of increases in wild boar-vehicle accidents 2003–2016 in Sweden on personal injuries and costs. Safety 4(4):53
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Keuling O, Stier N, Roth M (2008) How does hunting influence activity and spatial usage in wild boar Sus scrofa L. Eur J Wildl Res 54:729–737
Keuling O, Leus K (2019) Sus scrofa. The IUCN Red List of Threatened Species 2019: e.T41775A44141833. https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T41775A44141833.en
Larter NC, Gates CC (1994) Home range size of wood bison: effects of age, sex and forage availability. J Mammal 75:142–149
Lazzaro L, Foggi B, Benesperi R (2015) Monitoraggio degli impatti di Capra hircus sulla vegetazione a Montecristo: relazione tecnica Azione C8. Technical Report for Life Project NAT/IT/000471 “Island Conservation in Tuscany, Restoring Habitat not Only for Birds”
Lemel J, Truvé J, Söderberg B (2003) Variation in ranging and activity behaviour of European wild boar (Sus scrofa) in Sweden. Wildl Biol 9:29–36
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
MacKenzie DI, Nichols JD, Royle A, Pollock KH, Bailey LL, Hines JE (2017) More than two occupancy states. In: Occupancy estimation and modelling. Inferring patterns and dynamics of species occurrence, Chapter 9, 2nd edn. Academic press, Cambridge, pp 377–397
Maestre FT, Cortina J (2004) Are Pinus halepensis plantations useful as a restoration tool in semiarid Mediterranean areas? Forest Ecol Manag 198:303–317
Måren IE, Karki S, Prajapati C, Yadav RK, Shrestha BB (2015) Facing north or south: Does slope aspect impact forest stand characteristics and soil properties in a semiarid trans-Himalayan valley? J Arid Environ 121:112–123
Massei G, Genov P (1995) Preliminary analysis of factors influencing habitat-use by the wild boar. Ibex J Mount Ecol 3:168–170
Massei G, Genov PV (2004) The environmental impact of Wild Boar. Galemys 16:135–145
Massei G, Roy S, Bunting R (2011) Too many hogs? A review of methods to mitigate impact by wild boar and feral pigs. Hum Wildl Interact 5:79–99
Massei G, Kindberg J, Licoppe A, Gačić D, Šprem N, Kamler J, Baubet E, Hohmann U, Monaco A, Ozoliņš J, Cellina S, Podgórski T, Fonseca C, Markov N, Pokorny B, Ro-sell C, Náhlik A (2014) Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag Sci 71:492–500
Mazzerolle MJ (2019) Model selection and multimodel inference based on (Q)AIC(c). R package version 2.2–2. https://cran.r-project.org
Meredith M, Ridout M (2014) Overlap: estimates of coefficient of overlapping for animal activity patterns R package version 0.2.3. https://cran.r-project.org
Meriggi A, Lombardini M, Milanesi P, Brangi A, Lamberti P, Giannini F (2015) Management of wild boar in protected areas: the case of Elba Island. In: Angelici FM 2016 (Eds) Problematic Wildlife. A cross-disciplinary approach. Springer, New York
Monaco A, Carnevali L, Toso S (2010) Linee guida per la gestione del Cinghiale (Sus scrofa) nelle aree protette. 2° edizione. Quad. Cons. Natura, 34, Min. Ambiente – ISPRA. Technical report. (In Italian)
Mori E, Benatti L, Lovari S, Ferretti F (2017) What does the wild boar mean to the wolf? Eur J Wildl Res 63(1):9
Oberosler V, Groff C, Iemma A, Pedrini P, Rovero F (2017) The influence of human disturbance on occupancy and activity patterns of mammals in the Italian Alps from systematic camera trapping. Mamm Biol 87:50–61
Oldfield CA, Evans JP (2016) Twelve years of repeated wild hog activity promotes population maintenance of an invasive clonal plant in a coastal dune ecosystem. Ecol Evol 6:2569–2578
Podgórski Bas G, Jedrzejewska B, Sönnichsen L, Stanislaw S, Jedrzejewski W, Okarma H (2012) Spatiotemporal behavioural plasticity of wild boar (Sus scrofa) under constraining conditions of human pressure: primeval forest and metropolitan area. J Mammal 94(1):109–119
QGIS Development Team (2019) QGIS geographic information system. Open-source Geospatial Foundation Project. http://qgis.osgeo.org
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 14:322–337
Rovero F, Zimmermann F, Berzi D, Meek P (2013) Which camera trap type and how many do I need? A review of camera features and study designs for a range of wildlife research applications. Hystrix 24(2):148–156
Rodrigues P, Herreo J, Guarcía-Serrano A, Prada C, Giménez-Anaya A, Ayala R, Fernández Arberas O, Fonseca C (2016) Habitat use by wild boars Sus scrofa in Moncayo Nature Park, Spain. Pirineos 171:1–7
Russell JC, Meyer JY, Holmes ND, Pagad S (2017) Invasive alien species on islands: impacts, distribution, interactions and management. Environ Conserv 44(4):359–370
Schley L, Dufrêne M, Krier A, Frantz AC (2008) Patterns of crop damage by wild boar Sus scofa in Luxembourg over 10-year period. Eur J Wildl Res 54:589–599
Selva N, Jedrzejewska B, Jedrzejewski W, Wajrak A (2005) Factors affecting carcass use by a guild of scavengers in European temperate woodland. Can J For Res 83(12):1590–1601
Sendom C, Hughes J (2012) Rewilding the Scottish Highlands: do wild boars, Sus scrofa, usa a suitable foraging strategy to be effective ecosystem engineers? Restor Ecol 21(3):336–343
Serra G, Melega L, Baccetti N (2001) Piano d’azione nazionale per il Gabbiano corso (Larus audouinii). Quad. Cons. Natura, 6, Min. Ambiente – Ist. Naz. Fauna Selvatica. Technical report. (In Italian)
Siemann E, Carrillo JA, Gabler C, Zipp R, Rogers WE (2009) Experimental test of the impacts of feral hogs on forest dynamics and processes in the southeaster US. For Ecol Manag 258(5):546–553
Sollmann R (2018) A gentle introduction to camera-trap data analyses. Afr J Ecol 56:740–749
Toïgo C, Servantly S, Gaillard JM, Brandt S, Baubet E (2008) Disentangling natural from hunting mortality in an intensively hunted wild boar population. J Wildlife Manage 72(7):1532–1539
Torres-Porras J, Fernández-Llario P, Carranza J, Mateos C (2015) Conifer plantations negatively affect density of wild boars in Mediterranean ecosystem. Folia Zool 64(1):25–31
Tuscan Archipelago National Park (TANP) (2010) Capitolato tecnico per il servizio di prelievo ungulati nel Parco Nazionale Arcipelago Toscano periodo 2018–2020. CIG 746127788E. Technical Report. Available at: https://www.islepark.it/attachments/article/1351/Capitolato%20tecnico.pdf (In Italian)
Wirthner S, Schuetz M, Page-Dumroese DS, Busse M, Kirchner JW, Risch A (2012) Do changes in soil properties after rooting by wild boars (Sus scrofa) affect understory vegetation in Swiss hardwood forest? Can J For Res 45(3):585–592
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We sincerely thank the Tuscan Archipelago National Park and in particular Mrs Francesca Giannini and Francesca Puppo for the technical support. We are grateful to Silvia Miniati, Matteo Meriggi, Federico del Sala, and Sofia Gori for their contribution to the effort of data collection, along with Mr Lorenzo Petralia for field assistance. The research was funded by the Tuscan Archipelago National Park.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Handling editor: Juan Carranza.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Greco, I., Fedele, E., Salvatori, M. et al. Guest or pest? Spatio-temporal occurrence and effects on soil and vegetation of the wild boar on Elba island. Mamm Biol 101, 193–206 (2021). https://doi.org/10.1007/s42991-020-00083-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-020-00083-1