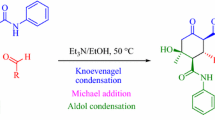
A robust method toward the synthesis of diastereomerically pure methyl (2S)-2-(1-benzyl-3-oxo-1,3-dihydro-2H-isoindol-2-yl)-3-methylbutanoate has been described. The key reactions in the synthesis are: HATU-mediated coupling, Pd-catalyzed Sonogashira coupling, base-mediated 5-exo-dig cyclization, and catalytic hydrogenation. The diastereomeric mixture is subjected to trituration with heptane to furnish both diastereomers in moderate yields. The relative stereochemistry was confirmed by the single crystal X-ray diffractometry. The key feature of the method is the simplicity of the diastereomeric separation.




Similar content being viewed by others
References
Valencia, E.; Freyer, A. J.; Shamma, M.; Fajardo, V. Tetrahedron Lett. 1984, 25, 599.
Pace, V.; Martínez, F.; Nova, C. I.; Fernández, M.; Sinisterra, J. V.; Alcántara, A. R. Tetrahedron Lett. 2009, 50, 3050.
(a) Furusaki, A; Hashiba, N.; Matsumoto, T.; Hirano, A.; Iwai, Y.; Omura, S. Bull. Chem. Soc. Jpn. 1982, 55, 3681. (b) Weinreb, S. M.; Garigipati, R. S.; Gainor, J. A. Heterocycles 1984, 21, 309.
Sorbera, L. A.; Leeson, P. A.; Silvestre, J.; Castaner, J. Drugs Future 2001, 26, 651.
Yagishita, F.; Ishikawa, H.; Onuki, T.; Hachiya, S.; Mino, T.; Sakamoto, M. Angew. Chem., Int. Ed. 2012, 51, 13023.
Björe, A.; Boström, J.; Davidsson, Ö.; Emtenäs, H.; Gran, H.; Iliefski, T.; Kajanus, J.; Olsson, R.; Sandberg, L.; Strandlund, G.; Sundell, J.; Yuan, Z.-Q. WO Patent 2008008022.
Mertens, A.; Zilch, H.; König, B.; Schäfer, W.; Poll, T.; Kampe, W.; Seidel, H. S.; Leser, U.; Leinert, H. J. Med. Chem. 1993, 36, 2526.
Maugeri, C.; Alisi, M. A.; Apicella, C.; Cellai, L.; Dragone, P.; Fioravanzo, E.; Florio, S.; Furlotti, G.; Mangano, G.; Ombrato, R.; Luisi, R.; Pompei, R.; Rincicotti, V.; Russo, V.; Vitiello, M.; Cazzolla, N. Bioorg. Med. Chem. 2008, 16, 3091.
Breytenbach, J. C.; van Dyk, S.; van den Heever, I.; Allin, S. M.; Hodkinson, C. C.; Northfield, C. J.; Page, M. I. Bioorg. Med. Chem. Lett. 2000, 10, 1629.
Zhao, X. Z.; Maddali, K.; Marchand, C.; Pommier, Y.; Burke, T. R., Jr. Bioorg. Med. Chem. 2009, 17, 5318.
Shirasaka, T.; Kunitake, T.; Tsuneyoshia, I. Brain Res. 2009, 1300, 105.
(a) Frank, A. J.; Man, H.-W.; Ge, C.; Saindane, M. US Patent 8415485. (b) Willems, H. M. G.; Kallblad, P.; Hardcastle, I. R.; Griffin, R. J.; Golding, B. T.; Lunec, J.; Noble, M. E. M.; Newell, D. R.; Calvert, A. H. US Patent 8258175. (c) Dally, R. D.; Woods, T. A. US Patent 20140275121.
(a) Zhao, P.-L.; Ma, W.-F.; Duan, A.-N.; Zou, M.; Yan, Y.-C.; You, W.-W.; Wu, S.-G. Eur. J. Med. Chem. 2012, 54, 813. (b) Ignasik, M.; Bajda, M.; Guzior, N.; Prinz, M.; Holzgrabe, U.; Malawska, B. Arch. Pharm. (Weinheim, Ger.) 2012, 345, 509. (c) Lindsley, C. W.; Conn, P. J.; Wood, M. R.; Hopkins, C. R.; Melancon, B. J.; Poslusney, M. S.; Engers, D. W. US Patent 20140288084. (d) Meltzer, H. Y.; Horiguchi, M. US Patent 8735397.
Guertin, K. R. US Patent 6482951.
Arakawa, K.; Nishimura, T.; Sugimoto, Y.; Takahashi, H.; Shimamura, T. US Patent 8362052.
McAlonan, H.; Murphy, J. P.; Nieuwenhuyzen, M.; Reynolds, K.; Sarma, P. K. S.; Stevenson, P.; Thompson, N. J. Chem. Soc., Perkin Trans. 1 2002, 69.
(a) Miyabe, H.; Yoshida, K.; Kobayashi, Y.; Matsumura, A.; Takemoto, Y. Synlett 2003, 1031. (b) Stájer, G.; Csende, F. Curr. Org. Chem. 2005, 9, 1277. (c) Buchert, M.; Meinke, S.; Prenzel, A. H. G. D.; Deppermann, N.; Maison, W. Org. Lett. 2006, 8, 5553.
(a) Donohoe, T. Sci. Synth. 2001, 10, 653. (b) Bonnett, R.; North, S. A. Adv. Heterocycl. Chem. 1981, 29, 341.
(a) Sundberg, R. J. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon Press: London, 1996, vol. 2, p. 119. (b) Bergman, J.; Janosik, T. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K., Eds.; Elsevier Science: Oxford, 2008, Vol. 3, p. 269.
(a) Fustero, S.; Herrera, L.; Lázaro, R.; Rodríguez, E.; Maestro, M. A.; Mateu, N.; Barrio, P. Chem.–Eur. J. 2013, 19, 11776. (b) Antico, P.; Capaccio, V.; di Mola, A.; Massa, A.; Palombi, L. Adv. Synth. Catal. 2012, 354, 1717. (c) Sović, I.; Stilinović, V.; Kaitner, B.; Kraljević-Pavelić, S.; Bujak, M.; Ĉuljak, K.; Novak, P.; Karminski-Zamola, G. J. Mol. Struct. 2011, 1006, 259. (d) Williams, F. J.; Jarvo, E. R. Angew. Chem., Int. Ed. 2011, 50, 4459. (e) Nieto, S.; Sayago, F. J.; Laborda, P.; Soler, T.; Cativiela, C.; Urriolabeitia, E. P. Tetrahedron 2011, 67, 4185. (f) Takizawa, S.; Inoue, N.; Hirata, S.; Sasai, H. Angew. Chem., Int. Ed. 2010, 49, 9725. (g) Enders, D.; Narine, A. A.; Toulgoat, F.; Bisschops, T. Angew. Chem., Int. Ed. 2008, 47, 5661. (h) Yao, T.; Larock, R. C. J. Org. Chem. 2005, 70, 1432. (i) Mancilla, T.; Carrillo, L.; Zamudio-Rivera, L. S.; Beltrán, H. I.; Farfán, N. Org. Prep. Proced. Int. 2001, 33, 341. (j) Allin, S. M.; Northfield, C. J.; Page, M. I.; Slawin, A. M. Z. Tetrahedron Lett. 1999, 40, 143.
(a) Gomes, P.; Araújo, M. J.; Rodrigues, M.; Vale, N.; Azevedo, Z.; Iley, J.; Chambel, P.; Morais, J.; Moreira, R. Tetrahedron 2004, 60, 5551. (b) Bolsakova, J.; Jirgensons, A. Chem. Heterocycl. Compd. 2017, 53, 1167. [Khim. Geterotsikl. Soedin. 2017, 53, 1167.] (c) Zubkov, F. I.; Boltukhina, E. V.; Krapivko, A. P.; Varlamov, A. V. Chem. Heterocycl. Compd. 2003, 39, 1534. [Khim. Geterotsikl. Soedin. 2003, 39, 1534.]
Scorzelli, F.; di Mola, A.; Palombi; L.; Massa A. Molecules 2015, 20, 8484.
Sagirova, Z. R.; Starodubtseva, E. V.; Turova, O. V.; Vinogradov, M. G. Russ. Chem. Bull., Int. Ed. 2012, 61, 1133. [Izv. Akad. Nauk, Ser. Khim. 2012, 1124.]
Viveros-Ceballos, J. L.; Cativiela, C.; Ordóñez, M. Tetrahedron: Asymmetry 2011, 22, 1479.
Chen, M.-D.; He, M.-Z.; Zhou, X.; Huang, L.-Q.; Ruan, Y.-P.; Huang, P.-Q. Tetrahedron 2005, 61, 1335.
Pérard-Viret, J.; Prangé, T.; Tomas, A.; Royer, J. Tetrahedron 2002, 58, 5103.
Bhat, S. V.; Nagasampagi, B. A.; Sivakumar, M. E. Chemistry of Natural Products; Narosa: Delhi, 2005, p. 317.
(a) Williams, R. M.; Burnett, C. M. In Asymmetric Synthesis and Application of α-Amino Acids; Soloshonok, V. A.; Izawa, K., Eds.; ACS: Washington, 2009, p. 420. (b) Kaiser, J.; Kinderman, S. S.; van Esseveldt, B. C. J.; van Delft, F. L.; Schoemaker, H. E.; Blaauw, R. H.; Rutjes, F. P. J. T. Org. Biomol. Chem. 2005, 3, 3435. (c) Rutjes, F. P. J. T.; Wolf, L. B.; Schoemaker, H. E. J. Chem. Soc., Perkin Trans. 1 2000, 4197. (d) Sardina, F. J.; Rapoport, H. Chem. Rev. 1996, 96, 1825.
(a) Vicario, J. L.; Badia, D.; Carrillo, L.; Reyes, E.; Etxebarria, J. Curr. Org. Chem. 2005, 9, 219. (b) Jarvo, E. R.; Miller, S. J. Tetrahedron 2002, 58, 2481. (c) Xu, L.-W.; Lu, Y. Org. Biomol. Chem. 2008, 6, 2047.
(a) Nájera, C.; Sansano, J. M. Chem. Rev. 2007, 107, 4584. (b) Maruoka, K.; Ooi, T. Chem. Rev. 2003, 103, 3013. (c) Chinchilla, R.; Nájera, C. In Asymmetric Synthesis and Application of α-Amino Acids; Soloshonok, V. A.; Izawa, K., Eds.; ACS: Washington, 2009, p. 251.
Tan, J.; Tong, Y.; Chen, Z. ChemistrySelect 2018, 3, 3886.
Brahmchari, D.; Verma, A. K.; Mehta, S. J. Org. Chem. 2018, 83, 3339.
Couty, S.; Liegault, B.; Meyer, C.; Cossy, J. Tetrahedron 2006, 62, 3882.
Bubar, A.; Estey, P.; Lawson, M.; Eisler, S. J. Org. Chem. 2012, 77, 1572.
Flessner, T.; Doye, S. J. Prakt. Chem. 1999, 341, 186.
Kuhakarn, C.; Kittigowittana, K.; Pohmakotr, M.; Reutrakul, V. ARKIVOC 2005, (i), 143.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschman, H. J. Appl. Crystallogr. 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(10), 1370–1374
Supplementary Information
ESM 1
(PDF 11256 kb)
Rights and permissions
About this article
Cite this article
Nighot, D., Jain, A.K., Singh, M. et al. A Convenient 5-exo-dig Cyclization Route to Diastereomerically Pure Methyl (2S)-2-(1-benzyl-3-oxo-1,3-dihydro-2H-isoindol-2-yl)-3-methylbutanoate. Chem Heterocycl Comp 56, 1370–1374 (2020). https://doi.org/10.1007/s10593-020-02825-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02825-y