Abstract
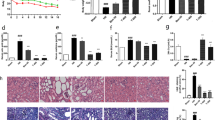
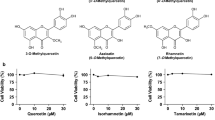
Isoorientin (ISO), a natural flavonoid compound, has been identified in several plants and its biological activity is determined and the study on lowering uric acid has not been reported. In view of the current status of treatment of hyperuricemia, we evaluated the hypouricemic effects of ISO in vivo and in vitro, and explored the underlying mechanisms. Yeast extract-induced hyperuricemia animal model as well as hypoxanthine and xanthine oxidase (XOD) co-induced high uric acid L-O2 cell model and enzymatic experiments in vitro were selected. The XOD activity and uric acid (UA) level were inhibited after the treatment of ISO in vitro and in vivo. Furthermore, serum creatinine (CRE) and blood urea nitrogen (BUN) levels were also significantly reduced and liver damage was recovered in pathological histology after the ISO administration in hyperuricemia animal model. The results of mechanism illustrated that protein expressions such as XOD, toll-like receptor 4 (TLR4), cathepsin B (CTSB), NLRP3, and its downstream caspase-1 as well as interleukin-18 (IL-18) were markedly downregulated by ISO intervention in vitro and in vivo. Our results suggest that ISO exerts a urate-lowering effect through inhibiting XOD activity and regulating TLR4-NLRP3 inflammasome signal pathway, thus representing a promising candidate therapeutic agent for hyperuricemia.
Graphic abstract
Both animal models and in vitro experiments suggested that ISO may effectively lower uric acid produce. The mechanism might be the inhibition of XOD activity and NLRP3 inflammasome of upregulation.







Similar content being viewed by others
Abbreviations
- ISO:
-
Isoorientin
- XOD:
-
Xanthine oxidase
- UA:
-
Uric acid
- CRE:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- TLR 4:
-
Toll-like receptor 4
- NF-κB:
-
Nuclear factor-κB
- CTSB:
-
Cathepsin B
- NLRP:
-
NOD-like receptor superfamily pyrin
- PRRs:
-
Pattern recognition receptors
- NLRC:
-
NOD-like receptor subfamily C
- NAIP:
-
NLR family, apoptosis inhibitory protein
- CARD:
-
Caspase activation and recruitment domain
- PYHIN:
-
Pyrin and HIN domain-containing protein
- URAT1:
-
Urate anion transporter 1
- GLUT9:
-
Glucose transporter 9
- OAT:
-
Organic cation transporter
- ABCG2:
-
ATP-binding cassette transporter ABCG2/BCRP
- ASC:
-
Apoptosis-associated speck-like protein
- IL-1β:
-
Interleukin-1β
- IL-18:
-
Interleukin-18
- IR:
-
Insulin resistance
- LPS:
-
Lipopolysaccharide
- K:
-
Potassium
- Ca:
-
Calcium
- FDA:
-
Food and Drug Administration
- BCA:
-
Bicinchoninic acid
- SPF:
-
Specific pathogen free
- PBS:
-
Phosphate-buffered saline
- PMSF:
-
Phenylmethylsulfonyl fluoride
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ROS:
-
Reactive oxygen species
- H&E:
-
Hematoxylin and eosin
- TGF-β:
-
Transforming growth factor beta
- RIPA:
-
Radioimmunoprecipitation
- PVDF:
-
Polyvinylidene fluoride
References
Chen C, Lu JM, Yao Q (2016) Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: an overview. Med Sci Monit 22:2501–2512
Wang H, Zhang H, Sun L, Guo W (2018) Roles of hyperuricemia in metabolic syndrome and cardiac-kidney-vascular system diseases. Am J Transl Res 10(9):2749–2763
Ali N, Perveen R, Rahman S, Mahmood S, Rahman S, Islam S, Haque T, Sumon AH, Kathak RR, Molla NH, Islam F, Mohanto NC, Nurunnabi SM, Ahmed S, Rahman M (2018) Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: a study on Bangladeshi adults. PLoS ONE 13(11):e0206850
Zhu Y, Pandya BJ, Choi HK (2011) Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheumatol 63(10):3136–3141
Kim Y, Kang J, Kim GT (2018) Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin Rheumatol 37(9):2529–2538
Liu R, Han C, Wu D, Xia X, Gu J, Guan H, Shan Z, Teng W (2015) Prevalence of hyperuricemia and gout in Mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int 2015:762820
Tan PK, Farrar JE, Gaucher EA, Miner JN (2016) Coevolution of URAT1 and uricase during primate evolution: implications for serum urate homeostasis and gout. Mol Biol Evol 33(9):2193–2200
Tan PK, Ostertag TM, Miner JN (2016) Mechanism of high affinity inhibition of the human urate transporter URAT1. Sci Rep 6:34995
Wang Z, Cui T, Ci X, Zhao F, Sun Y, Li Y, Liu R, Wu W, Yi X, Liu C (2019) The effect of polymorphism of uric acid transporters on uric acid transport. J Nephrol 32(2):177–187
Fujita K, Ichida K (2018) ABCG2 as a therapeutic target candidate for gout. Expert Opin Ther Targets 22(2):123–129
Desideri G, Castaldo G, Lombardi A, Mussap M, Testa A, Pontremoli R, Punzi L, Borghi C (2014) Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci 18(9):1295–1306
Ansari A, Aslam Z, De Sica A, Smith M, Gilshenan K, Fairbanks L, Marinaki A, Sanderson J, Duley J (2008) Influence of xanthine oxidase on thiopurine metabolism in Crohn’s disease. Aliment Pharmacol Ther 28(6):749–757
Aranda R, Domenech E, Rus AD, Real JT, Sastre J, Vina J, Pallardo FV (2007) Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic Res 41(11):1195–1200
Alem MM, Alshehri AM, Cahusac PM, Walters MR (2018) Effect of xanthine oxidase inhibition on arterial stiffness in patients with chronic heart failure. Clin Med Insights Cardiol 12:1179546818779584
El-Bassossy HM, Watson ML (2015) Xanthine oxidase inhibition alleviates the cardiac complications of insulin resistance: effect on low grade inflammation and the angiotensin system. J Transl Med 13:82
Ives A, Nomura J, Martinon F, Roger T, LeRoy D, Miner JN, Simon G, Busso N, So A (2015) Xanthine oxidoreductase regulates macrophage IL1beta secretion upon NLRP3 inflammasome activation. Nat Commun 6:6555
Bauernfeind F, Hornung V (2013) Of inflammasomes and pathogens–sensing of microbes by the inflammasome. EMBO Mol Med 5(6):814–826
Amores-Iniesta J, Barbera-Cremades M, Martinez CM, Pons JA, Revilla-Nuin B, Martinez-Alarcon L, Di Virgilio F, Parrilla P, Baroja-Mazo A, Pelegrin P (2017) Extracellular ATP activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep 21(12):3414–3426
Bai H, Yang B, Yu W, Xiao Y, Yu D, Zhang Q (2018) Cathepsin B links oxidative stress to the activation of NLRP3 inflammasome. Exp Cell Res 362(1):180–187
Amaral EP, Riteau N, Moayeri M, Maier N, Mayer-Barber KD, Pereira RM, Lage SL, Kubler A, Bishai WR, D’Imperio-Lima MR, Sher A, Andrade BB (2018) Lysosomal cathepsin release is required for NLRP3-inflammasome activation by Mycobacteriumtuberculosis in infected macrophages. Front Immunol 9:1427
Dalbeth N, Merriman TR, Stamp LK (2016) Gout. Lancet 388(10055):2039–2052
Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R (2005) TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol 174(8):5016–5023
Ghaemi-Oskouie F, Shi Y (2011) The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep 13(2):160–166
Grainger R, McLaughlin RJ, Harrison AA, Harper JL (2013) Hyperuricaemia elevates circulating CCL2 levels and primes monocyte trafficking in subjects with inter-critical gout. Rheumatology 52(6):1018–1021
Crisan TO, Cleophas MC, Oosting M, Lemmers H, Toenhake-Dijkstra H, Netea MG, Jansen TL, Joosten LA (2016) Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis 75(4):755–762
Chen M, Lu X, Lu C, Shen N, Jiang Y, Chen M, Wu H (2018) Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway. Arthritis Res Ther 20(1):20
Bakan A, Oral A, Elcioglu OC, Takir M, Kostek O, Ozkok A, Basci S, Sumnu A, Ozturk S, Sipahioglu M, Turkmen A, Voroneanu L, Covic A, Kanbay M (2015) Hyperuricemia is associated with progression of IgA nephropathy. Int Urol Nephrol 47(4):673–678
Gromadzinski L, Januszko-Giergielewicz B, Pruszczyk P (2015) Hyperuricemia is an independent predictive factor for left ventricular diastolic dysfunction in patients with chronic kidney disease. Adv Clin Exp Med 24(1):47–54
Lin MS, Dai YS, Pwu RF, Chen YH, Chang NC (2005) Risk estimates for drugs suspected of being associated with Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Intern Med J 35(3):188–190
Zemmez Y, Hjira N (2018) Allopurinol-induced DRESS syndrome: drug reaction with eosynophilia and systemic symptoms (DRESS). Pan Afr Med J 30:120
Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, Sidoroff A, Schneck J, Roujeau JC, Flahault A (2008) Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Investig Dermatol 128(1):35–44
Chohan S (2011) Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J Rheumatol 38(9):1957–1959
Yu KH (2007) Febuxostat: a novel non-purine selective inhibitor of xanthine oxidase for the treatment of hyperuricemia in gout. Recent Pat Inflamm Allergy Drug Discov 1(1):69–75
Bohm M, Vuppalanchi R, Chalasani N, Drug-Induced Liver Injury N (2016) Febuxostat-induced acute liver injury. Hepatology 63(3):1047–1049
Bruce SP (2006) Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout. Ann Pharmacother 40(12):2187–2194
Lopez-Lazaro M (2009) Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 9(1):31–59
Yan J, Zhang G, Hu Y, Ma Y (2013) Effect of luteolin on xanthine oxidase: inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem 141(4):3766–3773
Pauff JM, Hille R (2009) Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J Nat Prod 72(4):725–731
Lin X, Wei J, Chen Y, He P, Lin J, Tan S, Nie J, Lu S, He M, Lu Z, Huang Q (2016) Isoorientin from Gypsophila elegans induces apoptosis in liver cancer cells via mitochondrial-mediated pathway. J Ethnopharmacol 187:187–194
Yuan L, Wang J, Xiao H, Wu W, Wang Y, Liu X (2013) MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem Toxicol 53:62–68
Yuan L, Wei S, Wang J, Liu X (2014) Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. J Agric Food Chem 62(23):5390–5400
Yuan L, Wang Y, Wang J, Xiao H, Liu X (2014) Additive effect of zinc oxide nanoparticles and isoorientin on apoptosis in human hepatoma cell line. Toxicol Lett 225(2):294–304
Da Silva MM, De Assis AM, Da Rocha RF, Gasparotto J, Gazola AC, Costa GM, Zucolotto SM, Castellanos LH, Ramos FA, Schenkel EP, Reginatto FH, Gelain DP, Moreira JC (2013) Passiflora manicata (Juss.) aqueous leaf extract protects against reactive oxygen species and protein glycation in vitro and ex vivo models. Food Chem Toxicol 60:45–51
Yuan L, Wang J, Wu W, Liu Q, Liu X (2016) Effect of isoorientin on intracellular antioxidant defence mechanisms in hepatoma and liver cell lines. Biomed Pharmacother 81:356–362
Yuan L, Han X, Li W, Ren D, Yang X (2016) Isoorientin prevents hyperlipidemia and liver injury by regulating lipid metabolism, antioxidant capability, and inflammatory cytokine release in high-fructose-fed mice. J Agric Food Chem 64(13):2682–2689
Yuan L, Wu Y, Ren X, Liu Q, Wang J, Liu X (2014) Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-kappaB signaling pathway in BV-2 microglia. Mol Cell Biochem 386(1–2):153–165
Lee W, Ku SK, Bae JS (2014) Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vasc Pharmacol 62(1):13–14
Chen YX, Huang QF, Lin X, Wei JB (2013) Protective effect of isoorientin on alcohol-induced hepatic fibrosis in rats. Zhongguo Zhong Yao Za Zhi 38(21):3726–3730
Lin X, Chen Y, Lv S, Tan S, Zhang S, Huang R, Zhuo L, Liang S, Lu Z, Huang Q (2015) Gypsophila elegans isoorientin attenuates CCl(4)-induced hepatic fibrosis in rats via modulation of NF-kappaB and TGF-beta1/Smad signaling pathways. Int Immunopharmacol 28(1):305–312
Zhu SY, Zhou YD, Liu QS, Du GH (2007) Establishment and application of a high-throughput screening assay for xanthine oxidase inhibitor in vitro. Chin Pharm J 42(3):187–190
Chen GL, Sun XX, Wang QM, Zhang XM, Liu PH (2001) Study on hyperuricemia model in mice. Chin Pharm Bull 17(3):350–352
Niu YF, Liu K, Gao LH, Liu X, Li L (2015) Effects of 3,5,2’,4’-tetrahydroxychalcone on serum uric acid levels and the content of hepatic XOD/XDH in mice. Chin Pharm J 50(1):34–38
Xiong X, Ren Y, Cui Y, Li R, Wang C, Zhang Y (2017) Obeticholic acid protects mice against lipopolysaccharide-induced liver injury and inflammation. Biomed Pharmacother 96:1292–1298
Chen L, Li M, Wu JL, Li JX, Ma ZC (2019) Effect of lemon water soluble extract on hyperuricemia in a mouse model. Food Funct 10(9):6000–6008
Hayyan M, Hashim MA, AlNashef IM (2016) Superoxide ion: generation and chemical implications. Chem Rev 116(5):3029–3085
Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF (2000) Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci USA 97(20):10723–10728
Hou CW, Lee YC, Hung HF, Fu HW, Jeng KC (2012) Longan seed extract reduces hyperuricemia via modulating urate transporters and suppressing xanthine oxidase activity. Am J Chin Med 40(5):979–991
Qin Z, Wang S, Lin Y, Zhao Y, Yang S, Song J, Xie T, Tian J, Wu S, Du G (2018) Antihyperuricemic effect of mangiferin aglycon derivative J99745 by inhibiting xanthine oxidase activity and urate transporter 1 expression in mice. Acta Pharm Sin B 8(2):306–315
Hongyan L, Suling W, Weina Z, Yajie Z, Jie R (2016) Antihyperuricemic effect of liquiritigenin in potassium oxonate-induced hyperuricemic rats. Biomed Pharmacother 84:1930–1936
Shuyan Y, Mingsan M (2014) Interventional study of total flavone extract of lysimashia on hyperuricemia animal models. Henan University of Chinese Medicine, Zheng Zhou
Minami M, Ishiyama A, Takagi M, Omata M, Atarashi K (2005) Effects of allopurinol, a xanthine oxidase inhibitor, on renal injury in hypercholesterolemia-induced hypertensive rats. Blood Press 14(2):120–125
Dubreuil M, Zhu Y, Zhang Y, Seeger JD, Lu N, Rho YH, Choi HK (2015) Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis 74(7):1368–1372
Ma L, Hu J, Li J, Yang Y, Zhang L, Zou L, Gao R, Peng C, Wang Y, Luo T, Xiang X, Qing H, Xiao X, Wu C, Wang Z, He JC, Li Q, Yang S (2018) Bisphenol A promotes hyperuricemia via activating xanthine oxidase. FASEB J 32(2):1007–1016
Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, Castoldi A, Hiyane MI, Davanso MR, Latz E, Franklin BS, Kowaltowski AJ, Camara NO (2017) Soluble uric acid activates the NLRP3 inflammasome. Sci Rep 7:39884
He Y, Hara H, Nunez G (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41(12):1012–1021
Kim JJ, Jo EK (2013) NLRP3 inflammasome and host protection against bacterial infection. J Korean Med Sci 28(10):1415–1423
Funding
This work was supported by the Yunnan provincial key programs of Yunnan Eco-friendly Food International Cooperation Research Center project under Grant (2019ZG00904, 2019ZG00909), and the Science and Technology Plan Project of Yunnan Province (2018IA060).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
An, MF., Wang, MY., Shen, C. et al. Isoorientin exerts a urate-lowering effect through inhibition of xanthine oxidase and regulation of the TLR4-NLRP3 inflammasome signaling pathway. J Nat Med 75, 129–141 (2021). https://doi.org/10.1007/s11418-020-01464-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-020-01464-z