Abstract
Background
High levels of serum interleukin-6 (IL-6) correlate with disease severity in COVID-19. We hypothesized that tocilizumab (a recombinant humanized anti-IL-6 receptor) could improve outcomes in selected patients with severe worsening COVID-19 pneumonia and high inflammatory parameters.
Methods
The TOCICOVID study included a prospective cohort of patients aged 16–80 years with severe (requiring > 6 L/min of oxygen therapy to obtain Sp02 > 94%) rapidly deteriorating (increase by ≥ 3 L/min of oxygen flow within the previous 12 h) COVID-19 pneumonia with ≥ 5 days of symptoms and C-reactive protein levels > 40 mg/L. They entered a compassionate use program of treatment with intravenous tocilizumab (8 mg/kg with a maximum of 800 mg per infusion; and if needed a second infusion 24 to 72 h later). A control group was retrospectively selected with the same inclusion criteria. Outcomes were assessed at D28 using inverse probability of treatment weighted (IPTW) methodology.
Results
Among the 96 patients included (81% male, mean (SD) age: 60 (12.5) years), underlying conditions, baseline disease severity, and concomitant medications were broadly similar between the tocilizumab (n = 49) and the control (n = 47) groups. In the IPTW analysis, treatment with tocilizumab was associated with a reduced need for overall ventilatory support (49 vs. 89%, wHR: 0.39 [0.25–0.56]; p < 0.001). Albeit lacking statistical significance, there was a substantial trend towards a reduction of mechanical ventilation (31% vs. 45%; wHR: 0.58 [0.36–0.94]; p = 0.026). However, tocilizumab did not improve overall survival (wHR = 0.68 [0.31–1.748], p = 0.338). Among the 85 (89%) patients still alive at D28, patients treated with tocilizumab had a higher rate of oxygen withdrawal (82% vs. 73.5%, wHR = 1.66 [1.17–2.37], p = 0.005), with a shorter delay before being weaned of oxygen therapy (mean 11 vs. 16 days; p < 0.001). At D28, the rate of patients discharged from hospital was higher in the tocilizumab group (70% vs. 40%, wHR = 1.82 [1.22–2.75]; p = 0.003). The levels of CRP and fibrinogen post therapy (p < 0.001 for both variables) were significantly lower in the tocilizumab group (interaction test, mixed model). Rates of neutropenia (35% vs. 0%; p < 0.001) were higher in the tocilizumab group, yet rates of infections (22% vs. 38%, p = 0.089) including ventilator-acquired pneumonia (8% vs. 26%, p = 0.022) were higher in the control group.
Conclusion
These data could be helpful for the design of future trials aiming to counter COVID-19-induced inflammation, especially before patients require admission to the intensive care unit.
Similar content being viewed by others
Introduction
The pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection, leading to Coronavirus Disease-19 (COVID-19), is still unclear. Patients’ phenotypes are heterogeneous, and, in a subgroup of patients with life-threatening acute respiratory distress syndrome (ARDS), there is growing evidence that impaired interferon type I response and virally induced pro-inflammatory cytokines (including Interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF), and granulocyte-macrophage colony stimulating factor (GM-CSF)) lead to a cytokine release syndrome (CRS)-like hyperinflammatory stage at a secondary phase of the disease [1]. Moreover, pulmonary inflammation could trigger local vascular dysfunction and fibrinolysis, thereby contributing to fibrin deposition and alveolar-capillary blood-gas exchange dysfunction [2]. Hence, some authors have exhorted physicians to screen patients with severe COVID-19 for undue inflammation and suggested that immunosuppressive and/or immunomodulatory drugs should be investigated in this setting [3].
High levels of serum IL-6 have been reported in previous infections with human coronaviruses (i.e., SARS-CoV1 and Middle East respiratory syndrome-CoV) and correlate with disease severity in both SARS-CoV1 and SARS-CoV2 infections [4,5,6]. Yet, to date, it remains unclear whether IL-6 is the true driver of COVID-19–induced CRS-like or whether it could rather be the consequence of systemic inflammation steered by upstream cytokines, e.g., IL-1β or GM-CSF. Tocilizumab, a recombinant humanized anti-human IL-6 receptor (IL-6R) monoclonal antibody, is mainly used for the treatment of rheumatoid arthritis, but other indications include giant cell arteritis, idiopathic multicentric Castleman disease, and chimeric antigen receptor T cell therapy-induced cytokine release syndrome (a condition that to some extent can mimic the clinical picture of COVID-19–induced ARDS). Xu et al. first reported favorable outcomes in 21 Chinese patients treated with tocilizumab for severe COVID-19 pneumonia [7]. Moreover, Giamarellos-Bourboulis et al. demonstrated that the features of macrophage activation and immune dysregulation (including reduced HLA DR expression on CD14+ monocytes) induced by COVID-19 could be partially reversed in patients treated with tocilizumab [8].
In the emergency context of the COVID-19 pandemic, given the known safety profile of tocilizumab regarding the risk of viral infections [9], considering the limited access to ventilatory support, and pending the beginning of randomized controlled studies in our tertiary care center, we started an off-label compassionate use program of treatment with tocilizumab in highly selected patients with severe worsening COVID-19 pneumonia, hoping that it could defuse respiratory distress. The aim of this study is to investigate whether tocilizumab could indeed prevent respiratory worsening and the need for ventilatory support in this setting.
Methods
Study Design and Eligibility Criteria
The TOCICOVID study was performed in the context of an epidemic of a potentially life-threatening condition. The safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia was assessed by comparing outcomes of a prospective cohort of patients who entered a compassionate use program of tocilizumab (tocilizumab group) with those from a standard of care control group that did not receive tocilizumab (control group). As of March 21, 2020, given the exceptional sanitary circumstances resulting from the COVID-19 pandemic and the high fatality rates encountered, an off-label compassionate use program of tocilizumab endorsed by the COVID-19 crisis unit of our institution (Foch Hospital, Suresnes, Paris area, France) was proposed to highly selected patients hospitalized in medical wards who fulfilled all of the following eligibility criteria: (i) age 16–80 years; (ii) severe (i.e., requiring > 6 L/min of oxygen therapy to obtain SpO2 > 94%) rapidly deteriorating (i.e., increase by ≥3 L/min of oxygen flow within the previous 12 h) COVID-19 pneumonia (defined as either positive SARS-CoV2 reverse transcriptase polymerase chain reaction (RT-PCR) or typical aspect on lung CT scan (multiple ground-glass abnormalities with crazy paving, absence of either lymphadenopathy, and nodules); (iii) with ≥ 5 days of prior COVID-19–related symptoms; and (iv) C-reactive protein (CRP) levels > 40 mg/L (N < 5 mg/L). Exclusion criteria were the refusal to participate, active neoplastic disease with very limited life-expectancy, ongoing chemotherapy or another biological therapy, respiratory failure related to another cause than COVID-19 pneumonia, baseline leukocytes < 2.0 × 109 G/L, alanine transaminase/aspartate transaminase > 5 times the upper limit of normal or active HIV, HBV, or HBC infections.
The control group was identified retrospectively and consisted of unselected consecutive patients of both (i) Foch Hospital (who were mostly admitted before the compassionate use program of tocilizumab was started) and (ii) from a nearby (< 2.5 miles) distant hospital belonging to the same medical university (A. Paré hospital, Assistance Publique-Hôpitaux de Paris, Boulogne-Billancourt, France) before the latter center became active in a prospective randomized controlled trial assessing immune modulatory drugs for the treatment of COVID-19. Although they did not receive tocilizumab, patients from the control group fulfilled the same eligibility and exclusion criteria as those from the tocilizumab group.
Study Assessments
For each patient, baseline (meaning the time at which patients met eligibility criteria), epidemiological, demographic, clinical, and laboratory data as well as treatment modalities and outcomes were extracted by the investigators (MR, RP, JR, JEK, MG, FA) from electronic medical records using a standardized data collection form coded according to the local Institutional Review Board (IRB)-approved standards. Chest CT scans were blindly reviewed by two thoracic radiologists (ALB, MLC) who graded with a semi-quantitative scoring system the extent of COVID-19 pneumonia (< 10%, 10–25%, 25–50%, 50–75%, > 75% of parenchymal involvement). In case of discrepancies between evaluations, cases were further discussed until a consensus was reached.
Treatment Regimens
As of the beginning of the tocilizumab compassionate use program, a mobile team of clinical immunologists (MR, RP, JR, IM, MG, FA) could be reached daily (including weekends) in order to screen patients with rapidly deteriorating severe COVID-19 pneumonia and assess whether they met eligibility criteria. Nevertheless, the decision whether to start or not start treatment with tocilizumab was always taken collectively, after the discussion with both the patients (and, if needed, with their relatives) and their treating physicians. Tocilizumab (Hoffmann-La Roche Ltd., Basel, Switzerland) was administered as a first infusion of 8 mg/kg body weight (with a maximum of 800 mg per infusion), and, if respiratory symptoms did not improve after the first dose, a second infusion could be administered 24 to 72 h later (with the same treatment modalities) according to the physician’s judgment and again after the discussion with the mobile team of clinical immunologists. In both the tocilizumab and the control groups, best standard of care treatments (which could include the following: β-lactam antibiotics, intravenous ceftriaxone 1 g per day or amoxicillin/clavulanic acid 1 g tid for 7 days; azithromycin, 250 mg bid on day 1 and then 250 mg qid from days 2–5; hydroxychloroquine, 200 mg tid for 10 days; and lopinavir/ritonavir, 400/100 mg bid) were prescribed at the discretion of attending physicians (in compliance with local guidelines in force at the time of the study). Systemic corticosteroids could also be prescribed on a case-by-case basis. All patients received thromboprophylaxis according to French recommendations [10]. Oxygen therapy was administered through low-flow nasal cannula (≤ 6 L/min) or non-rebreather oxygen face masks (> 6 L/min). In both centers, patient management in the intensive care unit (ICU) complied with the French guidelines for the management of patients with COVID-19 (which were regularly updated over the study period) [11].
Outcomes
For all patients, outcomes were assessed at D28 (after baseline) by comparing follow-up data between the tocilizumab and the control groups. The main outcome was the need for ventilatory support (including high-flow nasal oxygen therapy and non-invasive and invasive mechanical ventilation). Secondary outcomes included the need for invasive mechanical ventilation; death from any cause; time before withdrawal of oxygen therapy; time to hospital discharge; modifications of the ordinal 8-category WHO R&D Blueprint scale levels (Supplementary Table S1); rates of pulmonary embolism; and modifications of biological parameters (including complete blood counts and serum CRP, fibrinogen, ferritin, and D-dimer levels) between baseline and D3 (range D2–D4), D7 (D5–D10), and D13 (D11–D15), when available. Safety outcomes included adverse events (graded on the CTCAE v5 scale) reported by physicians as possibly related to tocilizumab. Patient data was censored on May 9, 2020.
Statistical Analysis
Although there was no sample size calculation (given the lack of information regarding treatment effect at the time of the study design), the original total sample size of the prospective cohort study was arbitrarily set at 45 patients. In a classic two-arm trial, with 45 patients per group, there would be 90% power to detect a 40% risk reduction of the primary endpoint, at a two-sided significance level of α = 0.05 and assuming a 80% incidence of the primary endpoint. Outcomes were analyzed on an intention-to-treat basis (including all patients of the tocilizumab group who received at least one infusion of tocilizumab). Propensity score to estimate the probability that patients would be selected for tocilizumab or not was calculated using a multivariate logistic regression model to adjust for between-group differences in baseline characteristics based on covariates and factors. The inverse probability of treatment weighted (IPTW) methodology was performed to adjust for between-group differences, which were obtained from the propensity score [12]. Using the IPTW methodology approach, the weights for patients who received tocilizumab were set in proportion to the inverse of the propensity score; for the control group, the weights were set to the inverse of (1-propensity score). Time-dependent events were analyzed with unadjusted and weighted two-sided log-rank test and unadjusted and weighted two-sided hazard ratios (HR) with 95% confidence intervals (CI) based on a Cox proportional-hazards model and the associated Kaplan-Meier survival curves. Sensitive analyses were performed to assess the robustness of findings: We analyzed the unweighted samples and performed a trimmed analysis that was truncated at 5% of the extreme weights. The Bonferroni method was adopted to address the multiplicity issue in the Cox regression models. Categorical variables were presented as number and percentages; continuous variables were presented as mean and standard deviation (SD). Data were compared using Student’s t test or the Mann-Whitney test for continuous variables, and Fisher’s exact test or Pearson’s Chi-square test was used for categorical variables. A repeated-measures mixed-model testing group effect was used to analyze serum CRP, fibrinogen, ferritin levels, and complete blood count changes over time. Statistical significance was two-sided. p Values < 0.05 and < 0.01 were considered significant for the primary and the secondary outcomes, respectively. Analyses were performed using SAS software (version 9.4; SAS Institute, Carry, NC, USA).
Ethical Considerations
The TOCICOVID study was approved by the Foch IRB (approval number IRB00012437) and was registered on the National Institute of Health data platform INDS (n°4710280420). In accordance with the French legislation regarding institutional off-label drug evaluation (MR-004 study, as in the Official Journal of the French Republic n°0160, July 13, 2018), all patients in the tocilizumab group received oral and written information regarding tocilizumab and agreed to receive this treatment. Approval of the patients and decision to proceed with tocilizumab treatment were tracked in the patients’ medical files. No patients from both tocilizumab and control groups objected to the processing of their personal data.
Results
Patients
Between March 9 and April 11, 2020, among the 655 COVID-19 patients assessed for eligibility, 49 consecutive patients were included in the tocilizumab group (a single infusion, n = 34; while the remaining 15 patients—all of whom still required ≥ 9 L/min of oxygen therapy—had two), and 47 consecutive patients (Foch Hospital, n = 21; Ambroise Paré hospital, n = 26) fulfilling eligibility criteria were identified in the control group (Supplementary Fig. S1). Baseline, clinical, biological, and CT characteristics of the tocilizumab and control groups are presented in Table 1. Overall, the median duration of symptoms before study inclusion was 9 days, 78 (81%) were male, and their mean (SD) age was 60 (12.5) years. At baseline, all patients scored 4 on the 8-category WHO R&D Blueprint scale, thereby necessitating non-rebreathing masks but neither high-flow nasal oxygen therapy nor mechanical ventilation (whether non-invasive or invasive). Patients in the tocilizumab group required a higher oxygen flow at baseline to achieve a SpO2 > 94% (12 vs. 9 L/min; p = 0.011). Conversely, higher temperature grades (38.0 °C vs. 38.2 °C; p = 0.012), higher rates of treatment with lopinavir-ritonavir (2% vs. 24%; p = 0.001), and lower platelet counts (mean 225 vs. 186; p = 0.019) were reported in the control group. Of note, all other baseline characteristics (including concomitant medications) and the extent of COVID-19 pneumonia upon chest CT scan were similar between groups.
Propensity Score Model Development
Covariates included in the propensity score model were selected given the main identified risk factors for mortality in COVID-19 [4, 13, 14] identified at the time of the study design which could prompt physicians to start (or not start) treatment with tocilizumab. Such variables included the following: age; gender; obesity (defined by a body mass index ≥ 30 kg/m2); hypertension; cardiovascular disease; chronic kidney disease; chronic obstructive pulmonary disease; diabetes; immunosuppression (encompassing either HIV infection, ongoing treatment with conventional synthetic, targeted or biological disease modifying anti-rheumatic drugs, or ongoing treatment with > 10 mg/d of prednisone or equivalent); National Early Warning Score 2 score; CRP levels; lymphopenia (< 1 G/L); thrombocytopenia (< 150 G/L); and the use of early (within D5) high-dose (daily prednisone or equivalent dose > 0.5 mg/kg) corticosteroids (Supplementary Table S2). Propensity score ranged from 0.13 to 0.96 and from 0.03 to 0.82 in the tocilizumab and control groups, respectively (with 92.7% in the region of common support) (Fig. S2).
Outcomes
Comprehensive follow-up of the patients is shown on Fig. 1 (including after D28, when available). In the IPTW analysis, by comparison with the control group, treatment with tocilizumab was associated with a reduced need for overall ventilatory support (49 vs. 89%, wHR: 0.39 [0.25–0.56]; p < 0.001) within D28. Albeit lacking statistical significance, there was a clear trend towards a reduction of mechanical ventilation (31% vs. 45%; wHR: 0.58 [0.36–0.94]; p = 0.026). However, tocilizumab did not improve overall survival (wHR = 0.68 [0.31–1.748], p = 0.338). Among the 85 (89%) patients still alive at D28, patients treated with tocilizumab had a higher rate of oxygen withdrawal (82% vs. 73.5%, wHR = 1.782 [1.22–2.75], p = 0.003), with a shorter delay before being weaned of oxygen therapy (mean 11 vs. 16 days; p < 0.001) (Table 2). At D28, the rate of patients discharged from hospital was higher in the tocilizumab group (70% vs. 40%; p = 0.009). Sensitivity analyses provided consistent results (Supplementary Table S3). Kaplan-Meier survival curves for the main outcomes are presented in Fig. 2. Within D28, there were no differences between patients who received a single vs. two infusions of tocilizumab regarding either outcomes (data not shown). As expected, CRP levels dropped dramatically shortly after tocilizumab onset (< 40 mg/L and < 10 mg/L at D3 and D7, respectively, in all treated patients). Over the study period, the levels of both CRP and fibrinogen post-therapy (p < 0.001 for both variables) were significantly lower in the tocilizumab than in the control group (interaction test, mixed model). Conversely, the levels of ferritin and lymphocyte counts were similar between groups (Fig. 3). Full details of adverse events reported within D28 are reported in Supplementary Table S4. Of note, there were higher rates of neutropenia (35% vs. 0%, p < 0.001) in the tocilizumab group, yet there was a trend towards higher rates of bacterial infections (22% vs. 38%, p = 0.089; including ventilator-acquired pneumonia: 8% vs. 26%, p = 0.022) and shorter time to infection (mean 18 vs. 10 days, p = 0.029) in the control group. No fungal infection was reported. There were neither statistically significant differences regarding the rates of pulmonary embolism (16% vs. 11%, p = 0.117), grades 3–4 increase of alanine aminotransferase (25% vs. 6%, p = 0.374), nor grades 3–4 increase of aspartate aminotransferase (17% vs. 17%, p = 0.965).
Discussion
In the context of the COVID-19 pandemic and tensions worldwide regarding healthcare facilities, there is an urgent need for effective treatments likely to reduce the crunch of intensive care unit beds. Studies assessing the efficacy of antiviral drugs have shown mixed results [15,16,17,18,19,20], Conversely, there is growing evidence that virally induced hyperinflammation seems detrimental in a subgroup of patients with high inflammatory parameters [1, 3] and several studies are currently investigating immunomodulatory drugs for the treatment of COVID-19.
Here, in selected severe patients with rapidly deteriorating pneumonia and high inflammatory parameters, tocilizumab was associated with a reduced need for both non-invasive and invasive ventilation and thus seemed to prevent respiratory worsening. Since the need for invasive mechanical ventilation was previously shown to correlate with death [21], and although no difference was reported regarding mortality rates in the present series, larger-scale studies could hopefully demonstrate such an effect. Moreover, since older age is a major risk factor for mortality in COVID-19 [4, 13, 14], the fact that elderly patients were not included in this compassionate use program is likely to have scaled down the study power to detect such an effect on mortality. Next, the fact that tocilizumab significantly reduced the time to both oxygen withdrawal and hospital discharge is another significant finding likely to reduce the burden of COVID-19 on healthcare facilities. Although the rates of venous thromboembolic events were similar in both groups, the fact that tocilizumab significantly reduced fibrinogen is an encouraging sign, suggesting that tocilizumab might reduce fibrin deposition within infected lung tissue. Overall, tocilizumab was well tolerated, with no trend for increased septic complications despite higher rates of neutropenia. When monitored for SARS-CoV2, some patients showed evidence of viral clearance (negative second RT-PCR in 7 (33%) of the 21 tested patients) despite the use of this biological drug.
These results are in line with the first case series reported by both Chinese and Italian teams, which advocated that tocilizumab could be a promising treatment for COVID-19 [7, 22]. Moreover, further retrospective case-control studies suggested that this drug could prevent intensive care unit admission and reduce mortality rates [23,24,25,26], including in a retrospective study with propensity score matching involving 630 patients from the ICU [27]. Strikingly, in the latter study, tocilizumab decreases hospital-related mortality (HR: 0.64, 95%CI 0.47–0.87; p = 0.040). Conversely, reports of tocilizumab failure for COVID-19 have also been reported [28,29,30], and, in another study of 21 patients (matched to a historical control group using propensity score matching), such beneficial effect of tocilizumab was not outlined [31]. Yet, it should be emphasized that in the latter study, all patients received high-dose methylprednisolone —which might have skewed the study results—and the levels of oxygen therapy were not specified. Moreover, preliminary press releases from two randomized placebo-controlled trials involving tocilizumab and sarilumab (another monoclonal antibody targeting IL-6R) stated that these drugs failed to meet both their primary and key secondary endpoints, possibly due to broad inclusion criteria [32, 33]. Here, we hypothesized that tocilizumab could be mostly beneficial in selected patients with rapidly deteriorating pneumonia before they require ventilatory support. Conversely, since most patients with COVID-19 spontaneously recover, the optimal timing of treatment and the potential utility of combining (either concomitantly or successively) effective antiviral agents with tocilizumab remain to be determined. Moreover, a preliminary study suggested that patients with high levels of IL-6 were the most likely to benefit from tocilizumab [34]. Hence, future (including fundamental) studies should address the characteristics of the patients most likely to benefit from IL-6 blockade.
Besides IL-6, other cytokines likely to be involved in the pathophysiology of severe COVID-19 and CRS syndrome-like presentation can also be targeted. Anakinra is an IL-1R antagonist with a short half-life (thereby allowing prompt discontinuation if needed) that blocks both IL-1α and IL-1β (e.g., cytokines that are upstream of IL-6). Recently published case series and case-controlled studies with high-dose anakinra (up to 5 mg/kg bid intravenously) have shown promising results [35,36,37], and prospective randomized trials evaluating this drug for COVID-19 are ongoing. Likewise, encouraging preliminary results have been reported with both Janus-associated kinase 1/2 and GM-CSF inhibition [38, 39]. Last, systemic corticosteroids are potent anti-inflammatory drugs and could represent a cheaper alternative than disease-modifying antirheumatic drugs. Although two randomized failed to evidence a clinical benefit with low-dose hydrocortisone [40, 41], a 10-day course of dexamethasone increased the number of ventilator-free days over a 28-day period in a Brazilian randomized controlled trial involving 299 patients [42]. Moreover, with more than 2100 and 4300 patients receiving dexamethasone and standard of care respectively, the RECOVERY trial provides robust evidence demonstrating that dexamethasone (6 mg daily for 10 days) reduces 28-day mortality (age-adjusted rate ratio: 0.83; 95%CI 0.75 to 0.93; p < 0.001) [43], as also evidenced in a recent meta-analysis pooling data from seven randomized trials [44]. Of note, in the present study, the rates of patients who received corticosteroids were low and comparable between groups, and the same trends were reported when excluding such patients from analyses. Overall, the combination of glucocorticoids and biologics (as in the ongoing TOCIDEX trial: NCT04476979) warrants further evaluation.
This study has several limitations. First, both study groups were not strictly comparable, with notably a higher rate of patients treated with lopinavir/ritonavir in the control group (yet this drug failed to demonstrate clinical benefit in a randomized controlled trial) [15] and lower platelet counts in the control group. In order to increase the sample size of the control group and enhance the comparability of both groups, the control group was set up by merging patients from two different centers, which might have led to a selection bias. Yet, both centers are nearby hospitals belonging to the same medical university (with strong medical partnership and joint teaching and research programs); all patients from the control group were enrolled consecutively, and their baseline characteristics were broadly similar between both institutions (Supplementary Table S5). Although baseline characteristics tended to be similar between groups and that robust statistical techniques were used for adjustment, treatment was not randomly assigned, and potential unmeasured confounders (e.g., D-dimer levels—which were not included in the model owing to the retrospective design of the study and missing data) may have biased our results. Last, the optimal dosing regimen (a single vs. repeated infusions) of tocilizumab and whether this drug could also be beneficial in other (e.g., elderly, immunocompromised, or ICU-hospitalized) patients who were not included in this study is unknown.
In conclusion, in selected patients with rapidly deteriorating severe COVID-19 pneumonia, and by comparison with a control group of patients with similar baseline characteristics and disease severity, tocilizumab was associated with a reduced need for ventilatory support, and shorter time to both oxygen withdrawal and hospital discharge, with an acceptable safety profile. Although encouraging, these findings alone do not support the generalized prescription of tocilizumab, and the results of ongoing larger-scale prospective randomized-controlled trials investigating IL-6R or IL-6 blockade are needed in order to confirm these results. Last, these data could also be helpful for the design of future trials aiming to counter COVID-19-induced inflammation, especially before patients require admission to the ICU.
References
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8.
Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–40.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72(8):4410–5.
Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–6.
Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–5.
Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3.
Cañete JD, Hernández MV, Sanmartí R. Safety profile of biological therapies for treating rheumatoid arthritis. Expert Opin Biol Ther. 2017;17(9):1089–103.
Susen S, Tacquard CA, Godon A, Mansour A, Nguyen P, Godier A, et al. Traitement anticoagulant pour la prévention du risque thrombotique chez un patient hospitalisé avec COVID-19 et surveillance de l’hémostase propositions du GIHP et du GFHT. https://www.portailvasculaire.fr/sites/default/files/docs/covid-19_gihp-gfht-3_avril_final.pdf.
SRLF-SFAR-SFMU-GFRUP-SPILF-SPLF. Recommandations d’experts portant sur la prise en charge en réanimation des patients en période d’épidémie à SARS-CoV2. Avr. 2020. https://www.srlf.org/wp-content/uploads/2020/04/RFE-COVID_V4.pdf.
Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637–8.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.
Zhao X, Zhang B, Li P, Ma C, Gu J, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv. 2020;2020.03.17.20037572.
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–99.
Mahévas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844.
Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–704.
Pan H, Peto R, Abdool Karim Q, Alejandria M, Henao Restrepo AM, Hernandez Garcia C, et al. Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results. medRxiv. 2020;2020.10.15.20209817.
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–78.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;NEJMoa2007764:1813–26. https://doi.org/10.1056/NEJMoa2007764.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568.
Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–5.
Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–9.
Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–84.
Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020;ciaa954. https://doi.org/10.1093/cid/ciaa954.
Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–12.
Figuero-Pérez L, Olivares-Hernández A, Escala-Cornejo RA, Terán-Brage E, López-Gutiérrez Á, Cruz-Hernández JJ. Anakinra as a potential alternative in the treatment of severe acute respiratory infection associated with SARS-CoV-2 refractory to tocilizumab. Reumatol Clin. 2020;S1699-258X(20):30142–X. https://doi.org/10.1016/j.reuma.2020.06.003.
Innes AJ, Cook LB, Marks S, Bataillard E, Crossette-Thambiah C, Sivasubramaniam G, et al. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. Br J Haematol. 2020;190. https://doi.org/10.1111/bjh.16979.
Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158(1):e15–9.
Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8(5):695.
Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra® in hospitalised patients with severe COVID-19 associated pneumonia. 2020. Available at: https://www.roche.com/investors/updates/inv-update-2020-07-29.htm. Accessed October 10th 2020.
Sanofi provides update on Kevzara® (sarilumab) phase 3 trial in severe and critically ill COVID-19 patients outside the U.S. 2020. Available at: https://www.sanofigenzyme.com/about-us/newsroom/2020/2020-09-01-00-00-00. Accessed October 10th 2020.
Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, Marcos-Jiménez A, Sánchez-Alonso S, Fernández-Díaz C, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol. 2020;S0091–6749(20):31329–4. https://doi.org/10.1016/j.jaci.2020.09.018.
Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79(10):1381–2.
Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–400.
Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–31.
Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–146.e3.
De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2(8):e465–73.
Writing Committee for the REMAP-CAP Investigators, Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–29. https://doi.org/10.1001/jama.2020.17022.
Dequin P-F, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1–9. https://doi.org/10.1001/jama.2020.16761.
Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1–11. https://doi.org/10.1001/jama.2020.17021.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020;NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436.
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1–13. https://doi.org/10.1001/jama.2020.17023.
Acknowledgments
We are grateful to Louise Bouzeghoub, Chloé Delille, Louise Mimville, Arnaud Noysette, Kewin Panel, and Clara Duran for their help in data collection. We also thank Bernard Trillat (graphical support) and Jean-Marie Michot (MD, scientific counseling). We are grateful to Jacques Léglise (director), Brigitte Bonan (MD, pharmacy department), and Elisabeth Hulier-Ammar (PhD, clinical research department), Foch Hospital, for their support. We would like to express our gratitude to all patients who participated in this study, as well as to the healthcare providers who are involved on the frontline of this pandemic.
Funding
All costs (including funding for tocilizumab) were borne by Foch Hospital.
Author information
Authors and Affiliations
Consortia
Contributions
MR, AV, and FA conceptualized the study.
MR, RP, AV, JR, MB, ALB, CC, MLC, TC, MAC, EF, EF, GG, DK, IM, MP, AR, HS, YS, AGSL, CT, MV, AV, BZ, LJC, JEK, MG, and FA collected data.
MR, RP, and AV analyzed data.
MR and AV provided graphical support.
MG, RP, and JR drafted the manuscript.
All authors contributed to revision of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
MR: investigator of NCT04315298 trial which investigates the efficacy and safety of sarilumab (licensed by Sanofi) in hospitalized patients with COVID-19; non-financial support from Novartis Pharma SAS, Bristol Myers Squibb, and Swedish Orphan Biovitrum (outside the submitted work). HS: non-financial support from Oxyvie; grants for Foch Foundation, Fonds de dotation pour la recherche en santé respiratoire, and Philips Foundation (outside the submitted work). GG: non-financial support from Bard (outside the submitted work). YS: non-financial support from Astra Zeneca, Novartis Pharma, Bristol Myers Squibb, Sanofi Aventis France, Shire France, Chugai Pharma France, and Pfizer SAS (outside the submitted work). JLC: personal fees and non-financial support from Novartis, Boehringer Ingelheim, and Astra Zeneca; grants and other from LVL Air Liquide, outside the submitted work. JEK: none. MG: consulting fees from GlaxoSmithKline and Astra Zeneca (outside the submitted work). FA: investigator of NCT04315298 trial which investigates the efficacy and safety of sarilumab (licensed by Sanofi) in hospitalized patients with COVID-19. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Matthieu Groh and Félix Ackermann are senior co-authors
Electronic Supplementary Material
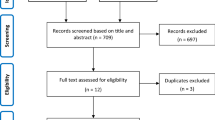
Fig S1
Flow chart showing the search strategy and inclusion/exclusion criteria for the study population. (PPTX 41 kb).
Fig S2
Propensity scores in the tocilizumab and control groups. ICU: intensive care unit; TCZ: tocilizumab. (PPTX 150 kb).
ESM 1
(DOCX 15 kb).
ESM 2
(DOCX 22 kb).
ESM 3
(DOCX 15 kb).
ESM 4
(DOCX 28 kb).
ESM 5
(DOCX 23 kb).
Rights and permissions
About this article
Cite this article
Roumier, M., Paule, R., Vallée, A. et al. Tocilizumab for Severe Worsening COVID-19 Pneumonia: a Propensity Score Analysis. J Clin Immunol 41, 303–314 (2021). https://doi.org/10.1007/s10875-020-00911-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-020-00911-6