Abstract
This study deals with early solidification of hypereutectic cast irons at varying carbon content and roughly constant alloying additions. Thermal analysis of such alloys shows that the start of the eutectic reaction occurs at a nearly constant temperature for mildly hypereutectic compositions. A similar trend is observed with more hypereutectic compositions but at a higher starting temperature. This jump in the start temperature of the eutectic reaction has not been previously evidenced and is here addressed by considering primary precipitation of graphite. Limiting the analysis to spheroidal graphite cast irons, it is demonstrated that simulation of primary graphite precipitation based on a 2D nucleation/lateral growth model allows substantiating the experimental distinction found between mildly and highly hypereutectic cast irons. This modeling explains that highly hypereutectic alloys start eutectic solidification in a limited temperature range that is nearly insensitive to the initial carbon equivalent of the alloy and to inoculation. This approach also suggests that the start of the eutectic solidification of mildly hypereutectic cast irons is shifted to lower temperature until growth of austenite enriches the liquid in carbon to such an extent that growth of graphite becomes possible.
Similar content being viewed by others
Notes
Owing to the simultaneous change in CE99, the liquidus lines when accounting for the addition of Ni are practically superimposed to those shown in Figure 2 and have thus not been plotted.
References
[1] A. Dioszegi, V.L. Diaconu and V. Fourlakidis: J. Therm. Anal. Calorim., 2016, Vol. 124, pp. 215–25.
[2] D.M. Stefanescu: Int. J. Metalcast., 2015, Vol. 9, pp. 7-22.
[3] H. Fredriksson and D.M. Stefanescu: Cast Iron Science and Technology, ASM International, OH, ASM Handbook, Vol. 1, 2017, pp. 81-86.
D.M. Stefanescu, R. Suarez, and Sung Bin Kim: China Foundry, 2020, Vol. 17, pp. 69–84.
S. Dawson and P. Popelar: Proceedings of the Keith Millis Symposium, AFS, 2013, pp. 59–66.
[6] M.D. Chaudhari, R.W. Heine and C.R. Loper: AFS Cast Metals Res. J., 1975, Vol. 11, pp. 52-60.
M. Hillert and V.V. Subba Rao: ISI Publ. 110, The Iron and Steel Institute, London (UK), 1969, pp. 204–12.
[8] S. Amini and R. Abbaschian: Carbon, 2013, Vol. 51, pp. 110-23.
[9] M.D. Chaudhari, R.W. Heine and C.R. Loper: AFS Trans., 1974, Vol. 82, pp. 379-86.
[10] P. Gustafsson: Scand. J. Metall., 1985, Vol. 14, pp. 259-67.
[11] R.W. Heine: AFS Trans., 1995, Vol. 103, pp. 199-206.
[12] M. Castro, M. Herrera, M.M. Cisneros, G. Lesoult and J. Lacaze: Int. J. Cast Met. Res., 1999, Vol. 11, pp. 369-74.
[13] J. Lacaze and B. Sundman: Metall. Trans., 1991, Vol. 22A, pp. 2211-23.
[14] J. Lacaze, G. Lesoult and M. Castro: Acta mater., 1998, Vol. 46, pp. 997-1010.
[15] K.M. Pedersen and N.S. Tiedje: Int. J. Cast Met. Res., 2007, Vol. 20, pp. 145-50.
M. Bjerre, N.S. Tiedje, J. Thorborg, and J.H. Hattel: IOP Conf. Ser.: Mater. Sci. Eng., 2015, Vol. 84, art. no. 012038.
[17] G. Lesoult M. Castro and J. Lacaze: Acta mater., 1998, Vol. 46, pp. 983-95.
[18] J.A. Dantzig and M. Rappaz: Solidification, Engineering Science, EPFL Press, Lausanne, Switzerland, 2009, pp. 268-75.
J. Lacaze, M. Castro, N. Aichoun, and G. Lesoult: Mem. Etudes Sci. Rev. Metall., 1989, pp. 85–97.
[20] M.A. Azeem, M.K. Bjerre, R.C. Atwood, N.S. Tiedje and P.D. Lee: Acta Mater., 2018, Vol. 155, pp. 393-401.
[21] J. Lacaze, J. Bourdie and M.J. Castro-Roman: Acta mater., 2017, Vol. 134, pp. 230-35.
[22] M. Coster and J.L. Chermant: Précis d’analyse d’images, Presses du CNRS, Paris, France, 1989, pp. 144-48.
[23] W.B. Hillig: Acta metall., 1966, Vol. 14, pp. 1868-69.
[24] D. Turnbull and J.C. Fisher: J. Chem. Phys., 1949, Vol. 17, pp. 71-73.
[25] J.W. Cahn, W.B. Hillig and G.W. Sears: Acta metall., 1964, Vol. 12, pp. 1421-39.
[26] K.A. Jackson, D.R. Uhlmann and J.D. Hunt: J. Crystal Growth, 1967, Vol. 1, pp. 1-36.
[27] W.P. Bosze and R. Trivedi: Metall. Trans., 1974, Vol. 5, pp. 511-12.
Acknowledgment
R.D.C.C. is pleased to acknowledge the support of Fundación Azterlan for performing his PhD research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted June 14, 2020.
Appendices
Appendix A
Tables A-I and A-II list the results selected from the works by Chaudhari et al.[6,9] Table A-I gives the reference of the alloys, their carbon, and silicon contents as well as the CE and CE99 values for industrial alloys.[9] For those alloys that have been spheroidized, the surface nodule count, NA, and nodularity are listed when they were reported. For alloys that solidified mostly in the stable system, the NA values were converted to volume number of graphite particles, NV, by means of \( N_{\text{V}} = \frac{2}{\pi } \cdot \frac{{N_{\text{A}} }}{{\bar{D}_{2} }} \), where \( \bar{D}_{2} \) is the average diameter of these particles in a 2D metallographic section.[22] For doing so, \( \bar{D}_{2} \) was evaluated by setting the area fraction of graphite to an average value ggra = 0.09: \( \bar{D}_{2} = \left( {\frac{4}{\pi } \cdot \frac{{g^{\text{gra}} }}{{N_{\text{A}} }}} \right)^{0.5} \). NV values are not given for alloys that showed essentially metastable solidification. Table A-II gives the same information for laboratory alloys.[6]
Appendix B
2.1 Classical Model[17]
The growth rate drgra/dt of a spherical particle of graphite of radius rgra is related to a carbon flux ϕ from the liquid through the following mass balance:
where \( w_{\text{C}}^{\text{gra}} \) and \( w_{\text{C}}^{i} \) are the carbon content in graphite and in the liquid at the liquid/graphite interface, respectively.
The transfer of carbon to a graphite precipitate proceeds through two steps in series: diffusion in the liquid, on the one hand, and interfacial reaction, on the other hand. Writing that the flux of carbon is the same for these two steps leads to the following equation:
where \( D_{\text{C}}^{l} \) is the carbon diffusion coefficient in the liquid, \( w_{\text{C}}^{l} \)is the carbon content in the liquid, K the interfacial kinetics constant and \( w_{\text{C}}^{\text{l/gra}} \) is the liquid carbon content at the equilibrium graphite liquidus.
Assuming a steady state carbon profile around the growing graphite nodule, Eq. [B-2] may be solved for \( w_{\text{C}}^{i} \)which is then inserted in Eq. [B-1] to give drgra/dt. Calculations were carried out with an initial nodule radius of 1 µm, \( D_{\text{C}}^{l} = 5 \cdot 10^{ - 9} \) m2 s−1 and K = 0.5 m s−1 as previously used.[14]
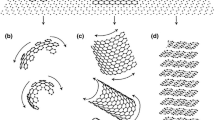
2.2 2D: Nucleation/Lateral Growth Model[21]
In this approach, a spherical shape is assumed which grows by continuous nucleation of new disk-shaped growth blocks at the outer surface of the spheroid on top of the so-called sectors, see Figure B1. The new blocks nucleate in epitaxy or semi-epitaxy with the underlying graphite, and then extend laterally along the surface. The overall growth direction thus remains parallel to the basal c crystallographic direction of graphite, while growth proceeds in the prismatic a direction along the outer surface of each sector.
The nucleation rate has been expressed according to Hillig.[23] As suggested by Turnbull and Fisher,[24] the fact that graphite precipitates from an alloy and not from a pure melt may be accounted for by multiplying the nucleation rate for pure melt by the atomic fraction of carbon, xC. After introduction of appropriate values for the parameters, the nucleation rate Ja was written:
where β corrects for structural factors and will be set to 1 as it should be for atoms such as carbon in Fe-C melts, as opposed to molecules for which it may assume higher values.[25] ξ is interface diffuseness which is 1 at most for a sharp interface.[25] In our previous study, a convenient value of ξ was found to be 0.1 which corresponds to an interface thickness of 1.6 atoms according to the computations by Jackson et al.[26] for the same case as Cahn et al.[25]
The overall growth rate of graphite was then described according to the poly-layer growth (PNG) model already used by Amini and Abbaschian[8] for describing thickening of lamellar graphite plates. The growth rate of a spheroid of radius rgra is thus given as:
where a is the distance between graphene layers, \( V_{\text{l}} \) is the lateral spreading rate of the ledge of the growth block and has been here assumed constant. Assuming this rate is controlled by diffusion of carbon in the liquid, the solution developed by Bosze and Trivedi[27] was adopted. This finally leads to:
in which, as stated above, β will be set to 1 and ξ to 0.1.
The value of xC that was used to calculate Ja in B-3 was 0.175 which corresponds to wC = 4.4 wt pct, i.e., a slightly hypereutectic alloy in the Fe-C binary system. The same atom fraction corresponds to a strongly hypereutectic alloy in the Fe-Si-C system. It was, however, decided to keep the same expression for Ja as a change of 1.5 at. pct (0.5 wt pct) of carbon leads to a change of about 10 pct of the pre-exponential factor in Eq. [B-5] leading to insignificant change in the result because of the exponential.
Rights and permissions
About this article
Cite this article
Castro-Román, M.J., Lacaze, J., Regordosa, A. et al. Revisiting Thermal Analysis of Hypereutectic Spheroidal Graphite Cast Irons. Metall Mater Trans A 51, 6373–6386 (2020). https://doi.org/10.1007/s11661-020-06005-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-020-06005-7