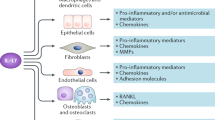
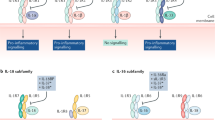
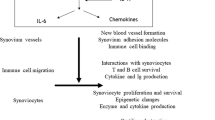
Abstract
In inflammatory rheumatic disorders, the immune system attacks and damages the connective tissues and invariably internal organs. During the past decade, remarkable advances having been made towards our understanding on the cellular and molecular mechanisms involved in rheumatic diseases. The discovery of IL-23/IL-17 axis and the delineation of its important role in the inflammation led to the introduction of many needed new therapeutic tools. We will present an overview of the rationale for targeting therapeutically the IL-23/IL-17 axis in rheumatic diseases and the clinical benefit which has been realized so far. Finally, we will discuss the complex interrelationship between IL-23 and IL-17 and the possible uncoupling in certain disease settings.


Similar content being viewed by others
References
Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365(22):2110–2121. https://doi.org/10.1056/NEJMra1100359
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055):2023–2038. https://doi.org/10.1016/S0140-6736(16)30173-8
McGonagle D, McDermott MF (2006) A proposed classification of the immunological diseases. PLoS Med 3(8):e297. https://doi.org/10.1371/journal.pmed.0030297
Nestle FO, Kaplan DH, Barker J (2009) Psoriasis. N Engl J Med 361(5):496–509. https://doi.org/10.1056/NEJMra0804595
Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet 390(10089):73–84. https://doi.org/10.1016/S0140-6736(16)31591-4
Lewis JE, Fu SM, Gaskin F (2013) Autoimmunity, end organ damage, and the origin of autoantibodies and autoreactive T cells in systemic lupus erythematosus. Discov Med 15(81):85–92
Tsokos GC (2020) Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol 21(6):605–614. https://doi.org/10.1038/s41590-020-0677-6
Chabaud M, Fossiez F, Taupin JL, Miossec P (1998) Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol 161(1):409–414
Miossec P, Kolls JK (2012) Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11(10):763–776. https://doi.org/10.1038/nrd3794
Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ (1995) Human IL-17: a novel cytokine derived from T cells. J Immunol 155(12):5483–5486
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6(11):1123–1132. https://doi.org/10.1038/ni1254
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6(11):1133–1141. https://doi.org/10.1038/ni1261
Gaffen SL, Jain R, Garg AV, Cua DJ (2014) The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14(9):585–600. https://doi.org/10.1038/nri3707
Iwakura Y, Ishigame H (2006) The IL-23/IL-17 axis in inflammation. J Clin Invest 116(5):1218–1222. https://doi.org/10.1172/JCI28508
Beringer A, Noack M, Miossec P (2016) IL-17 in chronic inflammation: From discovery to targeting. Trends Mol Med 22(3):230–241. https://doi.org/10.1016/j.molmed.2016.01.001
Theis KA, Roblin DW, Helmick CG, Luo R (2018) Prevalence and causes of work disability among working-age US adults 2011–2013 NHIS. Disabil Health J 11(1):108–115. https://doi.org/10.1016/j.dhjo.2017.04.010
Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA (2016) Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol 68(7):1582–1587. https://doi.org/10.1002/art.39692
Rachakonda TD, Schupp CW, Armstrong AW (2014) Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 70(3):512–516. https://doi.org/10.1016/j.jaad.2013.11.013
Saad J, Mathew D (2020) Nonsteroidal Anti-Inflammatory Drugs (NSAID) Toxicity. In: StatPearls. Treasure Island (FL)
Sostres C, Gargallo CJ, Lanas A (2013) Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther 15(Suppl 3):S3. https://doi.org/10.1186/ar4175
Shirota Y, Illei GG, Nikolov NP (2008) Biologic treatments for systemic rheumatic diseases. Oral Dis 14(3):206–216. https://doi.org/10.1111/j.1601-0825.2008.01440.x
Wellcome Trust Case Control C, Australo-Anglo-American Spondylitis C, Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davison D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop MG, Connell J, Dominiczak A, Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Biologics in RAG, Genomics Study Syndicate Steering C, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hilder SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Breast Cancer Susceptibility C, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Newport M, Sirugo G, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Davison D, Ferreira T, Pereira-Gale J, Hallgrimsdo’ttir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Brown MA, Compston A, Farrall M, Hall AS, Hattersley AT, Hill AV, Parkes M, Pembrey M, Stratton MR, Mitchell SL, Newby PR, Brand OJ, Carr-Smith J, Pearce SH, McGinnis R, Keniry A, Deloukas P, Reveille JD, Zhou X, Sims AM, Dowling A, Taylor J, Doan T, Davis JC, Savage L, Ward MM, Learch TL, Weisman MH, Brown M (2007) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39(11):1329–1337. https://doi.org/10.1038/ng.2007.17
Mells GF, Hirschfield GM (2015) Making the most of new genetic risk factors - genetic and epigenetic fine mapping of causal autoimmune disease variants. Clin Res Hepatol Gastroenterol 39(4):408–411. https://doi.org/10.1016/j.clinre.2015.05.002
Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJ, Shishkin AA, Hatan M, Carrasco-Alfonso MJ, Mayer D, Luckey CJ, Patsopoulos NA, De Jager PL, Kuchroo VK, Epstein CB, Daly MJ, Hafler DA, Bernstein BE (2015) Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518(7539):337–343. https://doi.org/10.1038/nature13835
Sarin R, Wu X, Abraham C (2011) Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci U S A 108(23):9560–9565. https://doi.org/10.1073/pnas.1017854108
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, Landewe R, Nash P, Pricop L, Yuan J, Richards HB, Mpofu S, Group FS (2015) Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 373(14):1329–1339. https://doi.org/10.1056/NEJMoa1412679
Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, Lin CY, Braun DK, Lee CH, Gladman DD, Group S-PS (2017) Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 76(1):79–87. https://doi.org/10.1136/annrheumdis-2016-209709
Deodhar A, Gottlieb AB, Boehncke WH, Dong B, Wang Y, Zhuang Y, Barchuk W, Xu XL, Hsia EC, Group CS (2018) Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 391(10136):2213–2224. https://doi.org/10.1016/S0140-6736(18)30952-8
Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, Shen YK, Szapary P, Randazzo B, Reich K (2015) A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 373(2):136–144. https://doi.org/10.1056/NEJMoa1501646
Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, Papp KA, Sofen H, Puig L, Foley P, Ohtsuki M, Flack M, Geng Z, Gu Y, Valdes JM, Thompson EHZ, Bachelez H (2018) Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 392(10148):650–661. https://doi.org/10.1016/S0140-6736(18)31713-6
Smolen JS, Agarwal SK, Ilivanova E, Xu XL, Miao Y, Zhuang Y, Nnane I, Radziszewski W, Greenspan A, Beutler A, Baker D (2017) A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis 76(5):831–839. https://doi.org/10.1136/annrheumdis-2016-209831
Blanco FJ, Moricke R, Dokoupilova E, Codding C, Neal J, Andersson M, Rohrer S, Richards H (2017) Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator- and placebo-controlled study. Arthritis Rheumatol 69(6):1144–1153. https://doi.org/10.1002/art.40070
Deodhar A, Gensler LS, Sieper J, Clark M, Calderon C, Wang Y, Zhou Y, Leu JH, Campbell K, Sweet K, Harrison DD, Hsia EC, van der Heijde D (2019) Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 71(2):258–270. https://doi.org/10.1002/art.40728
Baeten D, Ostergaard M, Wei JC, Sieper J, Jarvinen P, Tam LS, Salvarani C, Kim TH, Solinger A, Datsenko Y, Pamulapati C, Visvanathan S, Hall DB, Aslanyan S, Scholl P, Padula SJ (2018) Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis 77(9):1295–1302. https://doi.org/10.1136/annrheumdis-2018-213328
Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, Mpofu S, Richards HB, Group MS (2015) Secukinumab, an Interleukin-17A Inhibitor. Ankylosing Spondylitis N Engl J Med 373(26):2534–2548. https://doi.org/10.1056/NEJMoa1505066
van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, Van den Bosch F, Sieper J, Tomita T, Landewe R, Zhao F, Krishnan E, Adams DH, Pangallo B, Carliergroup C-Vs, H (2018) Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 392(10163):2441–2451. https://doi.org/10.1016/S0140-6736(18)31946-9
Sieper J, Poddubnyy D, Miossec P (2019) The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol 15(12):747–757. https://doi.org/10.1038/s41584-019-0294-7
Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ (2015) IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 21(7):719–729. https://doi.org/10.1038/nm.3895
Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D (2004) Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J 18(11):1318–1320. https://doi.org/10.1096/fj.03-1367fje
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13(5):715–725. https://doi.org/10.1016/s1074-7613(00)00070-4
Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421(6924):744–748. https://doi.org/10.1038/nature01355
Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ (2003) Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 198(12):1951–1957. https://doi.org/10.1084/jem.20030896
Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA (2001) Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol 166(12):7563–7570. https://doi.org/10.4049/jimmunol.166.12.7563
Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, Eberl G, Buckley CD, Kastelein RA, Pierce RH, Laface DM, Cua DJ (2012) IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med 18(7):1069–1076. https://doi.org/10.1038/nm.2817
Adamopoulos IE, Tessmer M, Chao CC, Adda S, Gorman D, Petro M, Chou CC, Pierce RH, Yao W, Lane NE, Laface D, Bowman EP (2011) IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol 187(2):951–959. https://doi.org/10.4049/jimmunol.1003986
Gaffen SL (2009) Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9(8):556–567. https://doi.org/10.1038/nri2586
Wynn TA (2005) T(H)-17: a giant step from T(H)1 and T(H)2. Nat Immunol 6(11):1069–1070. https://doi.org/10.1038/ni1105-1069
Hot A, Miossec P (2011) Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis 70(5):727–732. https://doi.org/10.1136/ard.2010.143768
Lubberts E (2015) The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol 11(10):562. https://doi.org/10.1038/nrrheum.2015.128
Beringer A, Miossec P (2019) Systemic effects of IL-17 in inflammatory arthritis. Nat Rev Rheumatol 15(8):491–501. https://doi.org/10.1038/s41584-019-0243-5
Robert M, Miossec P (2018) IL-17 in rheumatoid arthritis and precision medicine: from synovitis expression to circulating bioactive levels. Front Med (Lausanne) 5:364. https://doi.org/10.3389/fmed.2018.00364
Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, McClanahan T, Bowman EP (2010) Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther 12(1):R29. https://doi.org/10.1186/ar2936
Yago T, Nanke Y, Ichikawa N, Kobashigawa T, Mogi M, Kamatani N, Kotake S (2009) IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: A novel mechanism of osteoclastogenesis by IL-17. J Cell Biochem 108(4):947–955. https://doi.org/10.1002/jcb.22326
Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK (2010) Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 107(32):14292–14297. https://doi.org/10.1073/pnas.1009234107
Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD (2008) Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 9(2):166–175. https://doi.org/10.1038/ni1552
Majumder S, Amatya N, Revu S, Jawale CV, Wu D, Rittenhouse N, Menk A, Kupul S, Du F, Raphael I, Bhattacharjee A, Siebenlist U, Hand TW, Delgoffe GM, Poholek AC, Gaffen SL, Biswas PS, McGeachy MJ (2019) IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat Immunol 20(5):534–545. https://doi.org/10.1038/s41590-019-0367-4
McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ (2009) The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10(3):314–324. https://doi.org/10.1038/ni.1698
Li H, Hsu HC, Wu Q, Yang P, Li J, Luo B, Oukka M, Steele CH 3rd, Cua DJ, Grizzle WE, Mountz JD (2014) IL-23 promotes TCR-mediated negative selection of thymocytes through the upregulation of IL-23 receptor and RORgammat. Nat Commun 5:4259. https://doi.org/10.1038/ncomms5259
Koga T, Ichinose K, Kawakami A, Tsokos GC (2019) The role of IL-17 in systemic lupus erythematosus and its potential as a therapeutic target. Expert Rev Clin Immunol 15(6):629–637. https://doi.org/10.1080/1744666X.2019.1593141
Huffmeier U, Lascorz J, Bohm B, Lohmann J, Wendler J, Mossner R, Reich K, Traupe H, Kurrat W, Burkhardt H, Reis A (2009) Genetic variants of the IL-23R pathway: association with psoriatic arthritis and psoriasis vulgaris, but no specific risk factor for arthritis. J Invest Dermatol 129(2):355–358. https://doi.org/10.1038/jid.2008.233
Di Meglio P, Di Cesare A, Laggner U, Chu CC, Napolitano L, Villanova F, Tosi I, Capon F, Trembath RC, Peris K, Nestle FO (2011) The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS ONE 6(2):e17160. https://doi.org/10.1371/journal.pone.0017160
Uddin M, Codner D, Hasan SM, Scherer SW, O’Rielly DD, Rahman P (2015) Integrated genomics identifies convergence of ankylosing spondylitis with global immune mediated disease pathways. Sci Rep 5:10314. https://doi.org/10.1038/srep10314
Shao M, Xu S, Yang H, Xu W, Deng J, Chen Y, Gao X, Guan S, Xu S, Shuai Z, Pan F (2020) Association between IL-17A and IL-17F gene polymorphism and susceptibility in inflammatory arthritis: a meta-analysis. Clin Immunol 213:108374. https://doi.org/10.1016/j.clim.2020.108374
Song K, Liu L, Zhang X, Chen X (2020) An update on genetic susceptibility in lupus nephritis. Clin Immunol 210:108272. https://doi.org/10.1016/j.clim.2019.108272
Spach KM, Noubade R, McElvany B, Hickey WF, Blankenhorn EP, Teuscher C (2009) A single nucleotide polymorphism in Tyk2 controls susceptibility to experimental allergic encephalomyelitis. J Immunol 182(12):7776–7783. https://doi.org/10.4049/jimmunol.0900142
Gorman JA, Hundhausen C, Kinsman M, Arkatkar T, Allenspach EJ, Clough C, West SE, Thomas K, Eken A, Khim S, Hale M, Oukka M, Jackson SW, Cerosaletti K, Buckner JH, Rawlings DJ (2019) The TYK2-P1104A autoimmune protective variant limits coordinate signals required to generate specialized T cell subsets. Front Immunol 10:44. https://doi.org/10.3389/fimmu.2019.00044
Shaw MH, Boyartchuk V, Wong S, Karaghiosoff M, Ragimbeau J, Pellegrini S, Muller M, Dietrich WF, Yap GS (2003) A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc Natl Acad Sci U S A 100(20):11594–11599. https://doi.org/10.1073/pnas.1930781100
Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE (2014) The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev 13(4–5):496–502. https://doi.org/10.1016/j.autrev.2014.01.050
Jeon C, Sekhon S, Yan D, Afifi L, Nakamura M, Bhutani T (2017) Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Hum Vaccin Immunother 13(10):2247–2259. https://doi.org/10.1080/21645515.2017.1356498
Robert M, Miossec P (2020) Interleukin-17 and lupus: enough to be a target? For which patients? Lupus 29(1):6–14. https://doi.org/10.1177/0961203319891243
Silacci M, Lembke W, Woods R, Attinger-Toller I, Baenziger-Tobler N, Batey S, Santimaria R, von der Bey U, Koenig-Friedrich S, Zha W, Schlereth B, Locher M, Bertschinger J, Grabulovski D (2016) Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. MAbs 8(1):141–149. https://doi.org/10.1080/19420862.2015.1093266
Mease PJ, Genovese MC, Weinblatt ME, Peloso PM, Chen K, Othman AA, Li Y, Mansikka HT, Khatri A, Wishart N, Liu J (2018) Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol 70(11):1778–1789. https://doi.org/10.1002/art.40579
Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, Gottlieb AB, Assudani D, Bedford-Rice K, Coarse J, Ink B, McInnes IB (2020) Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 395(10222):427–440. https://doi.org/10.1016/S0140-6736(19)33161-7
Svecova D, Lubell MW, Casset-Semanaz F, Mackenzie H, Grenningloh R, Krueger JG (2019) A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin 17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol 81(1):196–203. https://doi.org/10.1016/j.jaad.2019.03.056
Chiricozzi A, De Simone C, Fossati B, Peris K (2019) Emerging treatment options for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis: evaluating bimekizumab and its therapeutic potential. Psoriasis (Auckl) 9:29–35. https://doi.org/10.2147/PTT.S179283
Weaver CT, Hatton RD, Mangan PR, Harrington LE (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852. https://doi.org/10.1146/annurev.immunol.25.022106.141557
Vignali DA, Kuchroo VK (2012) IL-12 family cytokines: immunological playmakers. Nat Immunol 13(8):722–728. https://doi.org/10.1038/ni.2366
Zhou L, Littman DR (2009) Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol 21(2):146–152. https://doi.org/10.1016/j.coi.2009.03.001
Ivanov II, Zhou L, Littman DR (2007) Transcriptional regulation of Th17 cell differentiation. Semin Immunol 19(6):409–417. https://doi.org/10.1016/j.smim.2007.10.011
Huh JR, Littman DR (2012) Small molecule inhibitors of RORgammat: targeting Th17 cells and other applications. Eur J Immunol 42(9):2232–2237. https://doi.org/10.1002/eji.201242740
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ (2017a) JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 16(12):843–862. https://doi.org/10.1038/nrd.2017.201
O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A (2013) Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 72(Suppl 2):ii111–ii115. https://doi.org/10.1136/annrheumdis-2012-202576
Leonard WJ, O’Shea JJ (1998) Jaks and STATs: biological implications. Annu Rev Immunol 16:293–322. https://doi.org/10.1146/annurev.immunol.16.1.293
Mogul A, Corsi K, McAuliffe L (2019) Baricitinib: the second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother 53(9):947–953. https://doi.org/10.1177/1060028019839650
Taylor PC (2019) Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford) 58(Suppl 1):i17–i26. https://doi.org/10.1093/rheumatology/key225
Fleischmann R (2017) A review of tofacitinib efficacy in rheumatoid arthritis patients who have had an inadequate response or intolerance to methotrexate. Expert Opin Pharmacother 18(14):1525–1533. https://doi.org/10.1080/14656566.2017.1370453
Wang F, Sun L, Wang S, Davis JM 3rd, Matteson EL, Murad MH, Luo F, Vassallo R (2020) Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin Proc 95(7):1404–1419. https://doi.org/10.1016/j.mayocp.2020.01.039
Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, Gelfand JM (2017) Psoriasis and comorbid diseases: implications for management. J Am Acad Dermatol 76(3):393–403. https://doi.org/10.1016/j.jaad.2016.07.065
Harden JL, Krueger JG, Bowcock AM (2015) The immunogenetics of psoriasis: a comprehensive review. J Autoimmun 64:66–73. https://doi.org/10.1016/j.jaut.2015.07.008
Hawkes JE, Yan BY, Chan TC, Krueger JG (2018) Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol 201(6):1605–1613. https://doi.org/10.4049/jimmunol.1800013
Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C (2018) Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int J Mol Sci 19 (2). doi:https://doi.org/10.3390/ijms19020530
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identificationof P, Associated ComorbidiTy project t, M (2013) Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 133(2):377–385. https://doi.org/10.1038/jid.2012.339
Ogdie A, Weiss P (2015) The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 41(4):545–568. https://doi.org/10.1016/j.rdc.2015.07.001
Rouzaud M, Sevrain M, Villani AP, Barnetche T, Paul C, Richard MA, Jullien D, Misery L, Le Maitre M, Aractingi S, Aubin F, Joly P, Cantagrel A, Ortonne JP, Beylot-Barry M (2014) Is there a psoriasis skin phenotype associated with psoriatic arthritis? Systematic literature review. J Eur Acad Dermatol Venereol 28(Suppl 5):17–26. https://doi.org/10.1111/jdv.12562
Rida MA, Chandran V (2020) Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol 214:108390. https://doi.org/10.1016/j.clim.2020.108390
Kim WB, Jerome D, Yeung J (2017) Diagnosis and management of psoriasis. Can Fam Physician 63(4):278–285
Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke WH, de Vlam K, Fiorentino D, Fitzgerald O, Gottlieb AB, McHugh NJ, Nash P, Qureshi AA, Soriano ER, Taylor WJ, Group for R, Assessment of P, Psoriatic A (2009) Treatment recommendations for psoriatic arthritis. Ann Rheum Dis 68(9):1387–1394. https://doi.org/10.1136/ard.2008.094946
Toussi A, Maverakis N, Le ST, Sarkar S, Raychaudhuri SK, Raychaudhuri SP (2020) Updated therapies for the management of psoriatic arthritis. Clin Immunol 220:108536. https://doi.org/10.1016/j.clim.2020.108536
Bell S, Nahle Z, Adamopoulos IE (2020) Psoriatic arthritis; overcoming the challenges by creating opportunities. Clin Immunol 218:108519. https://doi.org/10.1016/j.clim.2020.108519
Ritchlin CT, Colbert RA, Gladman DD (2017) Psoriatic Arthritis. N Engl J Med 376(10):957–970. https://doi.org/10.1056/NEJMra1505557
Eder L, Chandran V, Gladman DD (2015) What have we learned about genetic susceptibility in psoriasis and psoriatic arthritis? Curr Opin Rheumatol 27(1):91–98. https://doi.org/10.1097/BOR.0000000000000136
Bowes J, Ashcroft J, Dand N, Jalali-Najafabadi F, Bellou E, Ho P, Marzo-Ortega H, Helliwell PS, Feletar M, Ryan AW, Kane DJ, Korendowych E, Simpson MA, Packham J, McManus R, Brown MA, Smith CH, Barker JN, McHugh N, FitzGerald O, Warren RB, Barton A (2017) Cross-phenotype association mapping of the MHC identifies genetic variants that differentiate psoriatic arthritis from psoriasis. Ann Rheum Dis 76(10):1774–1779. https://doi.org/10.1136/annrheumdis-2017-211414
Di Cesare A, Di Meglio P, Nestle FO (2009) The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 129(6):1339–1350. https://doi.org/10.1038/jid.2009.59
Prinz I, Sandrock I, Mrowietz U (2020) Interleukin-17 cytokines: Effectors and targets in psoriasis-A breakthrough in understanding and treatment. J Exp Med 217 (1). doi:10.1084/jem.20191397
Hile G, Kahlenberg JM, Gudjonsson JE (2020) Recent genetic advances in innate immunity of psoriatic arthritis. Clin Immunol 214:108405. https://doi.org/10.1016/j.clim.2020.108405
Filer C, Ho P, Smith RL, Griffiths C, Young HS, Worthington J, Bruce IN, Barton A (2008) Investigation of association of the IL12B and IL23R genes with psoriatic arthritis. Arthritis Rheum 58(12):3705–3709. https://doi.org/10.1002/art.24128
Valdimarsson H (2007) The genetic basis of psoriasis. Clin Dermatol 25(6):563–567. https://doi.org/10.1016/j.clindermatol.2007.08.010
Tomfohrde J, Silverman A, Barnes R, Fernandez-Vina MA, Young M, Lory D, Morris L, Wuepper KD, Stastny P, Menter A et al (1994) Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 264(5162):1141–1145. https://doi.org/10.1126/science.8178173
Wang A, Bai Y (2020) Dendritic cells: The driver of psoriasis. J Dermatol 47(2):104–113. https://doi.org/10.1111/1346-8138.15184
Chen L, Deshpande M, Grisotto M, Smaldini P, Garcia R, He Z, Gulko PS, Lira SA, Furtado GC (2020) Skin expression of IL-23 drives the development of psoriasis and psoriatic arthritis in mice. Sci Rep 10(1):8259. https://doi.org/10.1038/s41598-020-65269-6
Miossec P (2003) Interleukin-17 in rheumatoid arthritis: If T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum 48(3):594–601. https://doi.org/10.1002/art.10816
Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA, Orlow SJ, Manga P, Krueger JG (2013) IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: Potential relevance to psoriasis. J Invest Dermatol 133(12):2741–2752. https://doi.org/10.1038/jid.2013.237
Adamopoulos IE, Suzuki E, Chao CC, Gorman D, Adda S, Maverakis E, Zarbalis K, Geissler R, Asio A, Blumenschein WM, McClanahan T, De Waal MR, Gershwin ME, Bowman EP (2015) IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Ann Rheum Dis 74(6):1284–1292. https://doi.org/10.1136/annrheumdis-2013-204782
Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, Purdy D, Fitch E, Iordanov M, Blauvelt A (2009) Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 129(9):2175–2183. https://doi.org/10.1038/jid.2009.65
Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, Chimenti S, Krueger JG (2011) Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 131(3):677–687. https://doi.org/10.1038/jid.2010.340
Tomalin LE, Russell CB, Garcet S, Ewald DA, Klekotka P, Nirula A, Norsgaard H, Suarez-Farinas M, Krueger JG (2020) Short-term transcriptional response to IL-17 receptor-A antagonism in the treatment of psoriasis. J Allergy Clin Immunol 145(3):922–932. https://doi.org/10.1016/j.jaci.2019.10.041
McGonagle DG, McInnes IB, Kirkham BW, Sherlock J, Moots R (2019) The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis 78(9):1167–1178. https://doi.org/10.1136/annrheumdis-2019-215356
Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M, Group CPS (2007) A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 356(6):580–592. https://doi.org/10.1056/NEJMoa062382
Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, Bautista-Molano W, Boehncke WH, Campbell W, Cauli A, Espinoza LR, FitzGerald O, Gladman DD, Gottlieb A, Helliwell PS, Husni ME, Love TJ, Lubrano E, McHugh N, Nash P, Ogdie A, Orbai AM, Parkinson A, O’Sullivan D, Rosen CF, Schwartzman S, Siegel EL, Toloza S, Tuong W, Ritchlin CT (2016) Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 68(5):1060–1071. https://doi.org/10.1002/art.39573
Markham A (2017) Guselkumab: first global approval. Drugs 77(13):1487–1492. https://doi.org/10.1007/s40265-017-0800-7
Group U-S, Group U-S, Group U-S, Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, Reich K, Amato D, Ball SG, Braun DK, Cameron GS, Erickson J, Konrad RJ, Muram TM, Nickoloff BJ, Osuntokun OO, Secrest RJ, Zhao F, Mallbris L, Leonardi CL (2016) Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 375(4):345–356. https://doi.org/10.1056/NEJMoa1512711
Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, Papp K, Spelman L, Toth D, Kerdel F, Armstrong AW, Stingl G, Kimball AB, Bachelez H, Wu JJ, Crowley J, Langley RG, Blicharski T, Paul C, Lacour JP, Tyring S, Kircik L, Chimenti S, Callis Duffin K, Bagel J, Koo J, Aras G, Li J, Song W, Milmont CE, Shi Y, Erondu N, Klekotka P, Kotzin B, Nirula A (2015) Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 373(14):1318–1328. https://doi.org/10.1056/NEJMoa1503824
Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, Newmark R, Feng J, Erondu N, Nirula A (2014) Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 370(24):2295–2306. https://doi.org/10.1056/NEJMoa1315231
Armstrong AW, Read C (2020) Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 323(19):1945–1960. https://doi.org/10.1001/jama.2020.4006
Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, Menter A, Philipp S, Sofen H, Tyring S, Berner BR, Visvanathan S, Pamulapati C, Bennett N, Flack M, Scholl P, Padula SJ (2017) Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 376(16):1551–1560. https://doi.org/10.1056/NEJMoa1607017
Li X, Andersen KM, Chang HY, Curtis JR, Alexander GC (2020) Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann Rheum Dis 79(2):285–291. https://doi.org/10.1136/annrheumdis-2019-216102
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, Primdahl J, McGonagle DG, Aletaha D, Balanescu A, Balint PV, Bertheussen H, Boehncke WH, Burmester GR, Canete JD, Damjanov NS, Kragstrup TW, Kvien TK, Landewe RBM, Lories RJU, Marzo-Ortega H, Poddubnyy D, Rodrigues Manica SA, Schett G, Veale DJ, Van den Bosch FE, van der Heijde D, Smolen JS (2020) EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 79(6):700–712. https://doi.org/10.1136/annrheumdis-2020-217159
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ (2017b) JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 17(1):78. https://doi.org/10.1038/nrd.2017.267
Sabat R, Ouyang W, Wolk K (2014) Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov 13(1):21–38. https://doi.org/10.1038/nrd4176
Kvist-Hansen A, Hansen PR, Skov L (2020) Systemic treatment of psoriasis with JAK inhibitors: A review. Dermatol Ther (Heidelb) 10(1):29–42. https://doi.org/10.1007/s13555-019-00347-w
Colbert RA, Ward MM (2017) JAK inhibitors taking on psoriatic arthritis. N Engl J Med 377(16):1582–1584. https://doi.org/10.1056/NEJMe1709907
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, Kudlacz E, Wang C, Menon S, Hendrikx T, Kanik KS (2017) Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 377(16):1525–1536. https://doi.org/10.1056/NEJMoa1615977
Paik J, Deeks ED (2019) Tofacitinib: a review in psoriatic arthritis. Drugs 79(6):655–663. https://doi.org/10.1007/s40265-019-01091-3
O’Shea JJ, Gadina M (2019) Selective Janus kinase inhibitors come of age. Nat Rev Rheumatol 15(2):74–75. https://doi.org/10.1038/s41584-018-0155-9
Papp K, Gordon K, Thaci D, Morita A, Gooderham M, Foley P, Girgis IG, Kundu S, Banerjee S (2018) Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med 379(14):1313–1321. https://doi.org/10.1056/NEJMoa1806382
Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF (2013) How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat Med 19(7):822–824. https://doi.org/10.1038/nm.3260
Zhao Q (2020) Bispecific antibodies for autoimmune and inflammatory diseases: clinical progress to date. BioDrugs 34(2):111–119. https://doi.org/10.1007/s40259-019-00400-2
Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P (1999) Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 42(5):963–970. https://doi.org/10.1002/1529-0131(199905)42:5%3c963::AID-ANR15%3e3.0.CO;2-E
Smolen JS (2020) Insights into the treatment of rheumatoid arthritis: a paradigm in medicine. J Autoimmun 110:102425. https://doi.org/10.1016/j.jaut.2020.102425
Taams LS (2020) Interleukin-17 in rheumatoid arthritis: Trials and tribulations. J Exp Med 217 (3). doi:10.1084/jem.20192048
Du J, Wang X, Tan G, Liang Z, Zhang Z, Yu H (2020) The association between genetic polymorphisms of interleukin 23 receptor gene and the risk of rheumatoid arthritis: An updated meta-analysis. Clin Immunol 210:108250. https://doi.org/10.1016/j.clim.2019.108250
Manolova I, Ivanova M, Vasilev G, Stoilov R, Miteva L, Stanilova S (2020) Impact of IL12B polymorphisms on genetic susceptibility and IL-12p40 and IL-23 serum levels in rheumatoid arthritis. Immunol Invest 49(1–2):1–14. https://doi.org/10.1080/08820139.2019.1622561
Mo WX, Yin SS, Chen H, Zhou C, Zhou JX, Zhao LD, Fei YY, Yang HX, Guo JB, Mao YJ, Huang LF, Zheng WJ, Zhang W, Zhang JM, He W, Zhang X (2017) Chemotaxis of Vdelta2 T cells to the joints contributes to the pathogenesis of rheumatoid arthritis. Ann Rheum Dis 76(12):2075–2084. https://doi.org/10.1136/annrheumdis-2016-211069
Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, Ackermann JA, Seefried M, Kleyer A, Uderhardt S, Haugg B, Hueber AJ, Daum P, Heidkamp GF, Ge C, Bohm S, Lux A, Schuh W, Magorivska I, Nandakumar KS, Lonnblom E, Becker C, Dudziak D, Wuhrer M, Rombouts Y, Koeleman CA, Toes R, Winkler TH, Holmdahl R, Herrmann M, Bluml S, Nimmerjahn F, Schett G, Kronke G (2017) Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol 18(1):104–113. https://doi.org/10.1038/ni.3579
Fragoulis GE, Siebert S, McInnes IB (2016) Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med 67:337–353. https://doi.org/10.1146/annurev-med-051914-021944
Mankia K, Di Matteo A, Emery P (2020) Prevention and cure: The major unmet needs in the management of rheumatoid arthritis. J Autoimmun 110:102399. https://doi.org/10.1016/j.jaut.2019.102399
Pavelka K, Chon Y, Newmark R, Lin SL, Baumgartner S, Erondu N (2015) A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. J Rheumatol 42(6):912–919. https://doi.org/10.3899/jrheum.141271
van Baarsen LG, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH, Gerlag DM, Tak PP (2014) Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: Possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther 16(4):426. https://doi.org/10.1186/s13075-014-0426-z
Pastor-Fernandez G, Mariblanca IR, Navarro MN (2020) Decoding IL-23 Signaling Cascade for New Therapeutic Opportunities. Cells 9 (9). doi:10.3390/cells9092044
Long D, Chen Y, Wu H, Zhao M, Lu Q (2019) Clinical significance and immunobiology of IL-21 in autoimmunity. J Autoimmun 99:1–14. https://doi.org/10.1016/j.jaut.2019.01.013
Burns LA, Maroof A, Marshall D, Steel KJA, Lalnunhlimi S, Cole S, Catrina A, Kirkham B, Taams LS (2020) Presence, function, and regulation of IL-17F-expressing human CD4(+) T cells. Eur J Immunol 50(4):568–580. https://doi.org/10.1002/eji.201948138
Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, Watanabe A, Origasa H, Shoji T, Sakamaki Y, van der Heijde D, Miyasaka N, Koike T (2014) Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: The J-RAPID randomized, placebo-controlled trial. Mod Rheumatol 24(5):715–724. https://doi.org/10.3109/14397595.2013.864224
Yue C, You X, Zhao L, Wang H, Tang F, Zhang F, Zhang X, He W (2010) The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int 30(12):1553–1557. https://doi.org/10.1007/s00296-009-1179-x
Genovese MC, Weinblatt ME, Aelion JA, Mansikka HT, Peloso PM, Chen K, Li Y, Othman AA, Khatri A, Khan NS, Padley RJ (2018) ABT-122, a bispecific dual variable domain immunoglobulin targeting tumor necrosis factor and interleukin-17A, in patients with rheumatoid arthritis with an inadequate response to methotrexate: a randomized. Double-Blind Study Arthritis Rheumatol 70(11):1710–1720. https://doi.org/10.1002/art.40580
Winthrop KL (2017) The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 13(4):234–243. https://doi.org/10.1038/nrrheum.2017.23
Kerschbaumer A, Sepriano A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, McInnes IB, Bijlsma JWJ, Burmester GR, de Wit M, Falzon L, Landewe R (2020) Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 79(6):744–759. https://doi.org/10.1136/annrheumdis-2019-216656
Harigai M (2019) Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford) 58(Suppl 1):i34–i42. https://doi.org/10.1093/rheumatology/key287
Dougados M (2020) Treat to target in axial spondyloarthritis: from its concept to its implementation. J Autoimmun 110:102398. https://doi.org/10.1016/j.jaut.2019.102398
Gravallese EM, Schett G (2018) Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 14(11):631–640. https://doi.org/10.1038/s41584-018-0091-8
DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA (2009) HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum 60(9):2633–2643. https://doi.org/10.1002/art.24763
Wendling D, Prati C, Chouk M, Verhoeven F (2020) Effects of anti-IL-23 and anti-IL-17: the hidden side of spondyloarthritis polymorphism? Joint Bone Spine 87(1):5–7. https://doi.org/10.1016/j.jbspin.2019.06.012
Chen S, Blijdorp I, van Mens L, Bowcutt R, Latuhihin T, van de Sande M, Shaw S, Yeremenko N, Baeten D (2020) IL-17A and IL-17F expression and functional responses in rheumatoid arthritis and peripheral spondyloarthritis. J Rheumatol. https://doi.org/10.3899/jrheum.190571
Erdes S, Nasonov E, Kunder E, Pristrom A, Soroka N, Shesternya P, Dubinina T, Smakotina S, Raskina T, Krechikova D, Povarova T, Plaksina T, Gordeev I, Mazurov V, Reshetko O, Zonova E, Eremeeva A, Chernyaeva E, Makulova T, Ivanov R (2020) Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults. Clin Exp Rheumatol 38(1):27–34
van der Heijde D, Gensler LS, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A, Farmer MK, Baeten D, Goldammer N, Coarse J, Oortgiesen M, Dougados M (2020) Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 79(5):595–604. https://doi.org/10.1136/annrheumdis-2020-216980
Mease P (2019) Ustekinumab fails to show efficacy in a phase III axial spondyloarthritis program: The importance of negative results. Arthritis Rheumatol 71(2):179–181. https://doi.org/10.1002/art.40759
Baeten DL, Kuchroo VK (2013) How cytokine networks fuel inflammation: interleukin-17 and a tale of two autoimmune diseases. Nat Med 19(7):824–825. https://doi.org/10.1038/nm.3268
Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464(7293):1371–1375. https://doi.org/10.1038/nature08949
Aden K, Rehman A, Falk-Paulsen M, Secher T, Kuiper J, Tran F, Pfeuffer S, Sheibani-Tezerji R, Breuer A, Luzius A, Jentzsch M, Hasler R, Billmann-Born S, Will O, Lipinski S, Bharti R, Adolph T, Iovanna JL, Kempster SL, Blumberg RS, Schreiber S, Becher B, Chamaillard M, Kaser A, Rosenstiel P (2016) Epithelial IL-23R signaling licenses protective IL-22 responses in intestinal inflammation. Cell Rep 16(8):2208–2218. https://doi.org/10.1016/j.celrep.2016.07.054
van der Heijde D, Deodhar A, Wei JC, Drescher E, Fleishaker D, Hendrikx T, Li D, Menon S, Kanik KS (2017) Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 76(8):1340–1347. https://doi.org/10.1136/annrheumdis-2016-210322
van der Heijde D, Baraliakos X, Gensler LS, Maksymowych WP, Tseluyko V, Nadashkevich O, Abi-Saab W, Tasset C, Meuleners L, Besuyen R, Hendrikx T, Mozaffarian N, Liu K, Greer JM, Deodhar A, Landewe R (2018) Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): Results from a randomised, placebo-controlled, phase 2 trial. Lancet 392(10162):2378–2387. https://doi.org/10.1016/S0140-6736(18)32463-2
Aringer M (2020) Inflammatory markers in systemic lupus erythematosus. J Autoimmun 110:102374. https://doi.org/10.1016/j.jaut.2019.102374
Frangou E, Georgakis S, Bertsias G (2020) Update on the cellular and molecular aspects of lupus nephritis. Clin Immunol 216:108445. https://doi.org/10.1016/j.clim.2020.108445
Martin JC, Baeten DL, Josien R (2014) Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin Immunol 154(1):1–12. https://doi.org/10.1016/j.clim.2014.05.004
Yang HX, Zhang W, Zhao LD, Li Y, Zhang FC, Tang FL, He W, Zhang X (2009) Are CD4+CD25-Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res Ther 11(5):R153. https://doi.org/10.1186/ar2829
Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC (2008) Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol 181(12):8761–8766. https://doi.org/10.4049/jimmunol.181.12.8761
Koga T, Ichinose K, Tsokos GC (2017) T cells and IL-17 in lupus nephritis. Clin Immunol 185:95–99. https://doi.org/10.1016/j.clim.2016.04.010
Dai H, He F, Tsokos GC, Kyttaris VC (2017) IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J Immunol 199(3):903–910. https://doi.org/10.4049/jimmunol.1700418
Lopez P, Rodriguez-Carrio J, Martinez-Zapico A, Perez-Alvarez AI, Benavente L, Caminal-Montero L, Suarez A (2020) IgM anti-phosphorylcholine antibodies associate with senescent and IL-17+ T cells in SLE patients with a pro-inflammatory lipid profile. Rheumatology (Oxford) 59(2):407–417. https://doi.org/10.1093/rheumatology/kez264
Vukelic M, Laloo A, Kyttaris VC (2020) Interleukin 23 is elevated in the serum of patients with SLE. Lupus:961203320952841. https://doi.org/10.1177/0961203320952841
Li M, Yang C, Wang Y, Song W, Jia L, Peng X, Zhao R (2020) The Expression of P2X7 receptor on Th1, Th17, and regulatory T cells in patients with systemic lupus erythematosus or rheumatoid arthritis and its correlations with active disease. J Immunol 205(7):1752–1762. https://doi.org/10.4049/jimmunol.2000222
Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC (2010) Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol 184(9):4605–4609. https://doi.org/10.4049/jimmunol.0903595
Sharabi A, Tsokos GC (2020) T cell metabolism: New insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol 16(2):100–112. https://doi.org/10.1038/s41584-019-0356-x
Li H, Adamopoulos IE, Moulton VR, Stillman IE, Herbert Z, Moon JJ, Sharabi A, Krishfield S, Tsokos MG, Tsokos GC (2020) Systemic lupus erythematosus favors the generation of IL-17 producing double negative T cells. Nat Commun 11(1):2859. https://doi.org/10.1038/s41467-020-16636-4
Hong H, Gao M, Wu Q, Yang P, Liu S, Li H, Burrows PD, Cua D, Chen JY, Hsu HC, Mountz JD (2020) IL-23 promotes a coordinated B cell germinal center program for class-switch recombination to IgG2b in BXD2 mice. J Immunol 205(2):346–358. https://doi.org/10.4049/jimmunol.2000280
Schmidt T, Paust HJ, Krebs CF, Turner JE, Kaffke A, Bennstein SB, Koyro T, Peters A, Velden J, Hunemorder S, Haag F, Steinmetz OM, Mittrucker HW, Stahl RA, Panzer U (2015) Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis Rheumatol 67(2):475–487. https://doi.org/10.1002/art.38955
Perry D, Sang A, Yin Y, Zheng YY, Morel L (2011) Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011:271694. https://doi.org/10.1155/2011/271694
van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, Werth VP, Gordon RM, Zhou B, Hsu B, Chevrier M, Triebel M, Jordan JL, Rose S (2018) Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392(10155):1330–1339. https://doi.org/10.1016/S0140-6736(18)32167-6
Wallace DJ, Furie RA, Tanaka Y, Kalunian KC, Mosca M, Petri MA, Dorner T, Cardiel MH, Bruce IN, Gomez E, Carmack T, DeLozier AM, Janes JM, Linnik MD, de Bono S, Silk ME, Hoffman RW (2018) Baricitinib for systemic lupus erythematosus: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 392(10143):222–231. https://doi.org/10.1016/S0140-6736(18)31363-1
Alunno A, Padjen I, Fanouriakis A, Boumpas DT (2019) Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: Integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells 8 (8). https://doi.org/10.3390/cells8080898
Murphy G, Isenberg DA (2019) New therapies for systemic lupus erythematosus - past imperfect, future tense. Nat Rev Rheumatol 15(7):403–412. https://doi.org/10.1038/s41584-019-0235-5
Wenzel J (2019) Cutaneous lupus erythematosus: New insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol 15(9):519–532. https://doi.org/10.1038/s41584-019-0272-0
Schwartz N, Stock AD, Putterman C (2019) Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 15(3):137–152. https://doi.org/10.1038/s41584-018-0156-8
Li Q, Wu H, Liao W, Zhao M, Chan V, Li L, Zheng M, Chen G, Zhang J, Lau CS, Lu Q (2018) A comprehensive review of immune-mediated dermatopathology in systemic lupus erythematosus. J Autoimmun 93:1–15. https://doi.org/10.1016/j.jaut.2018.07.007
Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA (2018) Bispecific antibodies: Design, therapy, perspectives. Drug Des Devel Ther 12:195–208. https://doi.org/10.2147/DDDT.S151282
Leonard WJ, Lin JX, O’Shea JJ (2019) The gammac family of cytokines: Basic biology to therapeutic ramifications. Immunity 50(4):832–850. https://doi.org/10.1016/j.immuni.2019.03.028
Schwartz DM, Bonelli M, Gadina M, O’Shea JJ (2016) Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 12(1):25–36. https://doi.org/10.1038/nrrheum.2015.167
Stark GR, Cheon H, Wang Y (2018) Responses to Cytokines and Interferons that Depend upon JAKs and STATs. Cold Spring Harb Perspect Biol 10 (1). https://doi.org/10.1101/cshperspect.a028555
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, H., Tsokos, G.C. IL-23/IL-17 Axis in Inflammatory Rheumatic Diseases. Clinic Rev Allerg Immunol 60, 31–45 (2021). https://doi.org/10.1007/s12016-020-08823-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-020-08823-4