Abstract
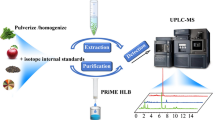
The toxic protein of ricin has drawn wide attention in recent years as a potential bioterrorism agent due to its high toxicity and wide availability. For the verification of the potential anti-terrorism activities, it is urgent for the quantification of ricin in food-related matrices. Here, a novel strategy of trypsin/Glu-C tandem digestion was introduced for quantitative detection of ricin marker peptides in several beverage matrices using isotope-labeled internal standard (IS)-mass spectrometry. The ricin in beverages was captured and enriched by biotinylated anti-ricin polyclonal antibodies conjugated to streptavidin magnetic beads. The purified ricin was cleaved using the developed trypsin/Glu-C tandem digestion method and then quantitatively detected by ultra-high-pressure liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) with isotope-labeled T7A and TG11B selected as IS. The use of trypsin/Glu-C digestion allows shorter peptides, which are more suitable for MS detection, to be obtained than the use of single trypsin digestion. Under the optimized tandem digestion condition, except for T7A in the A-chain, two resulting specific peptides of TG13A, TG28A from the A-chain and two of TG11B, TG33B from the B-chain were chosen as novel marker peptides with high MS response. The uniqueness of the selected marker peptides allows for unambiguous identification of ricin among its homologous proteins in a single run. The MS response of the four novel marker peptides is increased by more than 10 times compared with that of individual corresponding tryptic peptides. Both the marker peptides of A-chain T7A and B-chain TG11B were selected as quantitative peptides based on the highest MS response among the marker peptides from their individual chains. The limit of detection (LOD) of ricin is 0.1 ng/mL in PBS and 0.5 ng/mL in either milk or orange juice. The linear range of calibration curves for ricin were 0.5–300 ng/mL in PBS, 1.0–400 ng/mL in milk, and 1.0–250 ng/mL in orange juice. The method accuracy ranged between 82.6 and 101.8% for PBS, 88.9–105.2% for milk, and 95.3–118.7% for orange juice. The intra-day and inter-day precision had relative standard deviations (%RSD) of 0.3–9.4%, 0.7–8.9%, and 0.2–6.9% in the three matrices respectively. Furthermore, whether T7A or TG11B is used as a quantitative peptide, the quantitative results of ricin are consistent. This study provides not only a practical method for the absolute quantification of ricin in beverage matrices but also a new strategy for the investigation of illegal use of ricin in chemical weapon verification tasks such as OPCW biotoxin sample analysis exercises.






Similar content being viewed by others
References
Hartley MR, Lord JM. Cytotoxic ribosome-inactivating lectins from plants. Biochim Biophys Acta. 2004;1701(1–2):1–14.
Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004;44(4):371–83.
Jennifer Audi MB, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294(18):2342–451.
Worbs S, Skiba M, Bender J, Zeleny R, Schimmel H, Luginbühl W, et al. An international proficiency test to detect, identify and quantify ricin in complex matrices. Toxins. 2015;7(12):4987–5010.
Stirpe FB, M. G. Ribosome-inactivating proteins: progress and problems. Cell Mol Life Sci. 2006;63(16):1850–66.
McGrath SC, Schieltz DM, McWilliams LG, Pirkle JL, Barr JR. Detection and quantification of ricin in beverages using isotope dilution tandem mass spectrometry. Anal Chem. 2011;83(8):2897–905.
Shyu HF, Chiao DJ, Liu HW, Tang SS. Monoclonal antibody-based enzyme immunoassay for detection of ricin. Hybrid Hybridomics. 2002;21(1):69–73.
Godal A, Olsnes S, Pihl A. Radioimmunoassays of abrin and ricin in blood. J Toxicol Environ Health. 1981;8(3):409–17.
Campos AR, Gao Z, Blaber MG, Huang R, Schatz GC, Van Duyne RP, et al. Surface-enhanced Raman spectroscopy detection of ricin B chain in human blood. J Phys Chem C. 2016;120(37):20961–9.
Tang JJ, Sun JF, Lui R, Zhang ZM, Liu JF, Xie JW. New surface-enhanced Raman sensing chip designed for on-site detection of active ricin in complex matrices based on specific depurination. ACS Appl Mater Interfaces. 2016;8(3):2449–55.
Wang D, Baudys J, Barr JR, Kalb SR. Improved sensitivity for the qualitative and quantitative analysis of active ricin by MALDI-TOF mass spectrometry. Anal Chem. 2016;88(13):6867–72.
Sun J, Wang C, Shao B, Wang Z, Xue D, Liu Y, et al. Fast on-site visual detection of active ricin using a combination of highly efficient dual-recognition affinity magnetic enrichment and a specific gold nanoparticle probe. Anal Chem. 2017;89(22):12209–16.
Ostin A, Bergstrom T, Fredriksson S-A, Nilsson C. Solvent-assisted trypsin digestion of ricin for forensic identification by LC-ESI MS/MS. Anal Chem. 2007;79(16):6271–8.
Ma X, Tang J, Li C, Liu Q, Chen J, Li H, et al. Identification and quantification of ricin in biomedical samples by magnetic immunocapture enrichment and liquid chromatography electrospray ionization tandem mass spectrometry. Anal Bioanal Chem. 2014;406(21):5147–55.
Dupré M, Gilquin B, Fenaille F, Feraudet-Tarisse C, Dano J, Ferro M, et al. Multiplex quantification of protein toxins in human biofluids and food matrices using immunoextraction and high-resolution targeted mass spectrometry. Anal Chem. 2015;87(16):8473–80.
Fredriksson SA, Artursson E, Bergstrom T, Ostin A, Nilsson C, Astot C. Identification of RIP-II toxins by affinity enrichment, enzymatic digestion and LC-MS. Anal Chem. 2015;87(2):967–74.
Worbs S, Skiba M, Soderstrom M, Rapinoja ML, Zeleny R, Russmann H, et al. Characterization of ricin and R. communis agglutinin reference materials. Toxins. 2015;7(12):4906–34.
Sousa RB, Lima KSC, Santos CGM, Franca TCC, Nepovimova E, Kuca K, et al. A new method for extraction and analysis of ricin samples through MALDI-TOF-MS/MS. Toxins. 2019;11(4):201–22.
Feldberg L, Schuster O, Elhanany E, Laskar O, Yitzhaki S, Gura S. Rapid and sensitive identification of ricin in environmental samples based on lactamyl agarose beads using LC-MS/MS (MRM). J Mass Spectrom. 2020;55(1):e4482.
Cawley DB, Hedblom ML, Houston LL. Homology between ricin and Ricinus communie agglutinin: amino terminal sequence analysis and protein synthesis inhibition studies. Arch Biochem Biophys. 1978;190(2):744–55.
Araki T, Funatsu G. The complete amino acid sequence of the B-chain of ricin E isolated from small-grain castor bean seeds. Ricin E is a gene recombination product of ricin D and Ricinus communis agglutinin. Biochim Biophys Acta. 1987;911(2):191–200.
Kalb SR, Schieltz DM, Becher F, Astot C, Fredriksson SA, Barr JR. Recommended mass spectrometry-based strategies to identify ricin-containing samples. Toxins. 2015;7(12):4881–94.
Schieltz DM, McWilliams LG, Kuklenyik Z, Prezioso SM, Carter AJ, Williamson YM, et al. Quantification of ricin, RCA and comparison of enzymatic activity in 18 Ricinus communis cultivars by isotope dilution mass spectrometry. Toxicon. 2015;95:72–83.
Duriez E, Fenaille F, Tabet JC, Lamourette P, Ezan E. Detection of ricin in complex samples by immunocapture and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Proteome Res. 2008;7(9):4154.
Liu C-C, Liang L-H, Yang Y, Yu H-L, Yan L, Li X-S, et al. Direct acetonitrile-assisted trypsin digestion method combined with LC−MS/MS-targeted peptide analysis for unambiguous identification of intact ricin. J Proteome Res. 2020. https://doi.org/10.1021/acs.jproteome.0c00458.
OPCW Technical Secretariat. Guidelines for the fourth biotoxin sample analysis exercise. 2019-12. https://www.opcw.org/resources/documents/technical-secretariat/2019.
Funding
The work was supported by the State Key Laboratory of NBC Protection for Civilian (Grant No. SKLNBC2018-10).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of financial and nonfinancial interest.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the opinions of the State Key Laboratory of NBC Protection for Civilian or the Laboratory of Analytical Chemistry from the Research Institute of Chemical Defence.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2863 kb)
Rights and permissions
About this article
Cite this article
Liang, LH., Cheng, X., Yu, HL. et al. Quantitative detection of ricin in beverages using trypsin/Glu-C tandem digestion coupled with ultra-high-pressure liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 413, 585–597 (2021). https://doi.org/10.1007/s00216-020-03030-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-03030-8