Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1124
Peer-review started: May 24, 2020
First decision: August 22, 2020
Revised: August 29, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: October 26, 2020
Stem cells therapy could improve survival in patients with liver failure. Studies on stem cell therapy and related growth factors in decompensated cirrhosis has been on the forefront but has shown heterogenous results. Recent high-quality studies have shown a lack of efficacy and safety. Patients with acute-on-chronic liver failure (ACLF) are a unique group with high mortality in the short-term associated with rapid onset extrahepatic organ failures. In these patients, there is an urgent need to identify treatments that can improve liver cell function and mass, prevent sepsis/organ failure, ameliorate systemic inflammation, and increase transplant-free survival. Stem cells are a novel treatment in ACLF but with unclear efficacy and safety. In this narrative review, we discuss the basics of liver regeneration in patients with ACLF and update current clinical status of stem cell use in patients with ACLF for improving our understanding of future directions.
Core Tip: In patients with acute on chronic liver failure (ACLF), the efficacy and safety of stem cell therapy remains unclear because there are few adequately powered, high-quality studies. Most studies in ACLF have been performed in Asian centers and in those with hepatitis B virus infection. Some studies have demonstrated improved short-term survival as well as liver disease severity and better hepatic synthetic and excretory functions. However, long term clinical efficacy and safety as well as ideal protocols for stem cell extraction, dose, duration, route, and type, in patients with ACLF remain unclear and requires sufficiently powered studies.
- Citation: Philips CA, Augustine P. Still 'dwelling in the possibility' - critical update on stem cell therapy for acute on chronic liver failure. World J Stem Cells 2020; 12(10): 1124-1132
- URL: https://www.wjgnet.com/1948-0210/full/v12/i10/1124.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i10.1124
Acute-on-chronic liver failure (ACLF) is a distinct clinical syndrome characterized by liver failure in the presence of acute hepatic or non-hepatic injury in a patient with chronic liver disease. The acute insult leads to severe and persistent systemic inflammation followed by a cytokine storm resulting in rapid liver and extrahepatic organ failure, which is the hallmark of ACLF. Common acute injuries known to cause ACLF include alcoholic hepatitis, reactivation of chronic hepatitis B, acute non-hepatotropic and hepatotropic viral hepatitis, drug and herb-induced liver injury, and sepsis. Sepsis in particular can act as an acute insult as well as a consequence during the progression of ACLF that worsens organ failure. In contrast to compensated and decompensated cirrhosis, patients with ACLF are considered to have a very poor ‘hepatic reserve’. This reduction in hepatic reserve drives liver failure in the presence of persistent inflammation and immune dysregulation to further mitigate the regenerative potential of the hepatic mass under insult[1]. In this regard, management options in ACLF include treatment of acute injury (corticosteroids for alcoholic hepatitis, antiviral therapy for hepatitis B virus infection, and withdrawal of hepatotoxic drugs); modulation of immune functions (experimental use of granulocyte-colony stimulating factor, plasma exchange); management of severe inflammatory responses; reduction of circulating toxins (liver-assist devices); and supplementation of liver regenerative capacity (stem cell therapy or growth factor use). These practices can help reduce liver failure and its progression to multiple organ failure[2]. The augmentation of liver regeneration can be achieved through various forms of stem cell therapy or organ transplantation. In this review, we briefly discuss the pathophysiology of ACLF that substantiates stem cell use. We then explore the basics of stem cells and their contemporaneous use in the treatment of chronic liver failure and critically assess the current literature on various clinical studies on stem cell use in ACLF.
Cirrhosis is associated with progressive fibrosis, marked disruption of the hepatic-angioarchitecture, and blood flow leading to an increase in portal pressure and subsequent quantitative and qualitative hepatocyte loss. It is broadly divided into two phases: compensated and decompensated. Compensated cirrhosis also encompasses advanced compensated liver disease (ACLD) patients who have elastography-based liver stiffness values over 15 kPa along with clinically significant portal hypertension (hepatic venous pressure gradient ≥ 10 mmHg)[3]. This compensated phase may last for 10 to 15 years after which decompensation emerges with variceal bleeding, ascites, jaundice, and hepatic encephalopathy (HE).
The decompensated phase lasts 3 to 5 years after which the liver failure and multiple organ failure ensues leading to death or the need for a liver transplantation. The persistence of etiological mechanisms in patients with compensated cirrhosis leads to the continuation of liver cell necrosis and inflammation. These features lead to a reduction in liver parenchyma cells, increased resistance to the portal blood flow, worsening portal hypertension, and liver dysfunction[4]. An acute insult in the form of hepatic or non-hepatic liver injury in cirrhosis can lead to sudden deterioration in liver function complicated by extrahepatic organ failures that define ACLF.
According to the Asian Pacific Association for the Study of the Liver, ACLF is described as liver failure (serum bilirubin level of ≥ 5 mg per dL) and coagulopathy (an international normalized ratio of ≥ 1.5 or prothrombin activity of < 40%) that is complicated within four weeks by clinical ascites and/or encephalopathy in a patient with chronic liver disease or cirrhosis. According to the European Association for the Study of the Liver, ACLF is the development of acute decompensation (ascites, encephalopathy, gastrointestinal hemorrhage or bacterial infection) of cirrhosis complicated by either single organ failure (renal or otherwise) or multiple organ failures.
While a common definition has not been achieved between the East and West, liver failure plays a central role in the causation and progression of organ dysfunction in ACLF[5]. Hepatocyte regeneration can compensate for acute and chronic liver injury and depends on various signaling pathways that are contingent on cytokines and growth factors. The two-tier regenerative response showed that mature or adult hepatocytes replicate to sustain liver function in acute liver failure and normal physiological regeneration. In patients with ACLF, the proliferation of hepatic progenitor cells (HPCs) or liver stem cells initiates and regulate regenerative responses with the continued loss of mature hepatocytes[6].
Hepatic stellate cells (HepSCs) also have stem cell properties. Cytokines play a central role in driving ACLF and promoting organ failure. However, cytokines such as interleukin-6 (IL-6) and tumor-necrosis-factor alpha (TNF-α) have dual actions that can result in hepatocyte death but also enhance liver cell proliferation via activation of acute phase proteins with antiapoptotic effects. The tilt in balance between hepatocyte death or proliferation and regeneration depends on the underlying liver disease severity and intensity of acute insult. High representation of HPCs and intermediate hepatocytes (phenotype between progenitor cells or ductular cells and mature hepatocytes) that correlate with severity of underlying liver fibrosis and inflammation has been demonstrated in patients with ACLF. Activation of HPCs and HepSCs and associated cytokine signaling including hedgehog and Wingless and Int-1 (Wnt) pathways play the central role in hepatocyte mass maintenance in patients with ACLF[7]. In ACLF, an extensive ductular reaction occurs after intense acute insult and liver cell necrosis. This ductular proliferation involves mature cholangiocytes and ductular hepatocytes, the latter are located in the periphery of the portal tracts and proliferate and express cholangiocyte and hepatocyte markers. After an acute insult, various signaling cascades come into play to sustain quality and quantity of liver cell mass. Briefly, the important molecular cascade processes in liver regeneration during hepatic injury include the priming phase, the proliferation phase with complete (direct hepatotropic effect) and auxiliary (incomplete hepatotropic effect) cytokine and growth-factor driven ‘mitogen’ activity, and the termination phase[8].
Briefly, these phases include: (1) TNF-α mediated priming (through superfamily members and receptors) phase of liver regeneration through activation of the nuclear factor kB (NF-kB) transcription factor that is induced (through adaptor protein MyD88 through toll-like receptors) by intestine-derived lipopolysaccharide; (2) Activation of the complement component C5a for IL-6 induction and STAT3 pathway activation; (3) Activation of intercellular adhesion molecule 1 (ICAM-1) by leucocytes to activate Kupffer cells and subsequent beneficial cytokine signaling; (4) Lymphotoxin-alpha (LT-α) activation of TNF receptor 1 (TNFR1) in intrahepatic lymphocytes and lymphotoxin β receptor (LTβR) on the hepatocytes; (5) Secretion of stem cell factor and activation of its receptor, c-kit, within the liver microenvironment from bone marrow-derived sources that upregulate IL-6 to promote hepatocyte regeneration via HPCs activation; (6) Upregulation of hepatocyte growth factor (HGF) for activity via activation of the receptor tyrosine kinase c-Met; (7) Epidermal growth factor (EGF) family receptors and associated ligand activation resulting in the upregulation of transforming growth factor (TGF)-α and heparin-binding EGF (HB-EGF) that improve the mitogenic potential of existing hepatocytes; (8) Fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF, especially type A), and platelet-derived growth factor (PDGF)-related upregulation and mitogenic effect on sinusoidal endothelial cells and proliferation of liver cells; (9) Growth factor-related hepatocyte proliferation via Ras-MAPK signaling and phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathways; (10) Insulin-like growth factors (IGFs) type I and II, and their respective binding proteins (potent stimulators of hepatocyte mitogenesis); (11) Canonical Wnt pathway activation and signaling leading to stabilization and nuclear translocation of β-catenin, which performs transcriptional regulation leading to increased DNA synthesis within hepatocytes and mitogenesis; (12) Bone morphogenetic proteins (BMPs) activation in acute and chronic injury that induce hepatocyte proliferation; and (13) Bone marrow derived hematopoietic stem cells that became an important driver of liver cell regeneration.
Once proliferation is complete and satisfactory, the activin A (member of TGF superfamily) and TGF-β activate their individual receptor complexes on hepatocytes resulting in activation of SMAD2 and SMAD3 and inhibition of cell proliferation leading to termination of liver regeneration[9-11]. Progenitor cell mobilization for disease modification can be achieved via direct stem cell infusion or stimulation via systemic treatment with certain growth factors and cytokines such as the myeloid growth factor granulocyte- and granulocyte-macrophage-colony stimulating factors (G-CSF and GM-CSF) as well as interleukins IL-11, IL-3, and IL-8. Interleukin-22 (secreted by mesenchymal stem cells) was recently shown to ameliorate ACLF by reprogramming impaired regeneration pathways by upregulating STAT3, BCL2, Cyclin D, and antibacterial genes leading to hepatocyte proliferation[12]. In liver disease management, common stem cell treatments include direct progenitor cell infusion or growth factor-directed stimulation.
To summarize, in ACLF, hepatocyte replication occurs through activation and differentiation of HPCs (Figure 1). The TNF super family member 12 (known as TWEAK) and the TNF receptor family 12A (known as FN14) that are activated by macrophages, Kupffer cells, T cells, and interleukins and promote differentiation of HPCs into hepatocytes. Apoptosis and necrosis of hepatocytes activate the Wnt signaling (especially Wnt3) inhibiting the Notch-Hedgehog signaling with promotion of HPCs differentiation to hepatocytes. Activation and secretion of paracrine factors needed for hepatocyte growth and replication is supported by non-parenchymal cells of the liver (such as stellate cells) as well as the bone marrow. Acute liver injury leads to an increase in stromal cell derived factor 1 in bone marrow, stimulation and secretion of bone marrow derived hematopoietic stem cells, and epithelial progenitor cells that home towards the liver microenvironment where they upregulate hepatocyte replication and sinusoidal cell activation, which improve angiogenesis. The latter is a pre-requisite for liver regeneration.
Stem cells are undifferentiated cells that can continually proliferate. Stem cells are divided into embryonic, fetal, and adult types. The former can differentiate into any type of cell depending on the stimulus while the latter has a narrow variety of outcomes related to proliferation. Induced pluripotent stem cells and cord blood or amniotic fluid stem cells are other types. Based on the proliferation potential, stem cells can be further divided into totipotent, pluripotent, multipotent, oligopotent, or unipotent types. Stem cells can be autologous (obtained from self or patients themselves) or allogenic (from a donor). Adult stem cells have been the most utilized and studied among patients with advanced liver disease. The commonly sourced adult stem cells include hematopoietic stem cells (HemSC) that are isolated from blood, bone marrow, umbilical cord blood, or occasionally from peripheral blood based on expression of surface markers CD34+ and CD133+. Other examples include non-hematopoietic mesenchymal stem cells (MSC) that can be isolated from blood and multiple other tissues of the body and purely isolated bone-marrow mononuclear (BMMNC) or bone marrow stem (BMSC) cells. Adult stem cells such as the bone-marrow derived stem cells and HemSCs can be indirectly propagated through use of exogenous growth factors such as G-CSF or GM-CSF.
Direct stem cell use involves infusion into the portal vein, hepatic artery, splenic vein, hepatic sinusoids or percutaneous delivery, transplantation of clusters into the tissue component or the use of vectors[13-15]. Bone marrow-derived MSCs are the most commonly used source of multipotent cells for stem cell transplantation in experimental studies and clinical trials. According to the International Society for Cell Therapy, the minimal criteria required of derived MSCs include a fibroblastoid phenotype with expression of CD105, CD73, and CD90 and a lack of expression of CD45, CD34, CD14 (or CD11b), and CD79α (or CD19).
MSCs are easier to obtain including from adipose tissue. MSC express surface markers and liver specific genes including alpha fetoprotein, cytokeratin (CK)-18, CK-19, and hepatocyte nuclear factor and can easily differentiate into hepatocyte-like cells but with heterogenous and unstable functionality; hence, they are rarely used in human clinical trials[16]. Cytokines, chemokines, and growth factors secreted by adult stem cells (especially MSCs) are effective in reducing inflammation and hepatocyte apoptosis in both acute and chronic liver injury models. Similar assumptions have been made on their utility in patients with advanced liver cirrhosis.
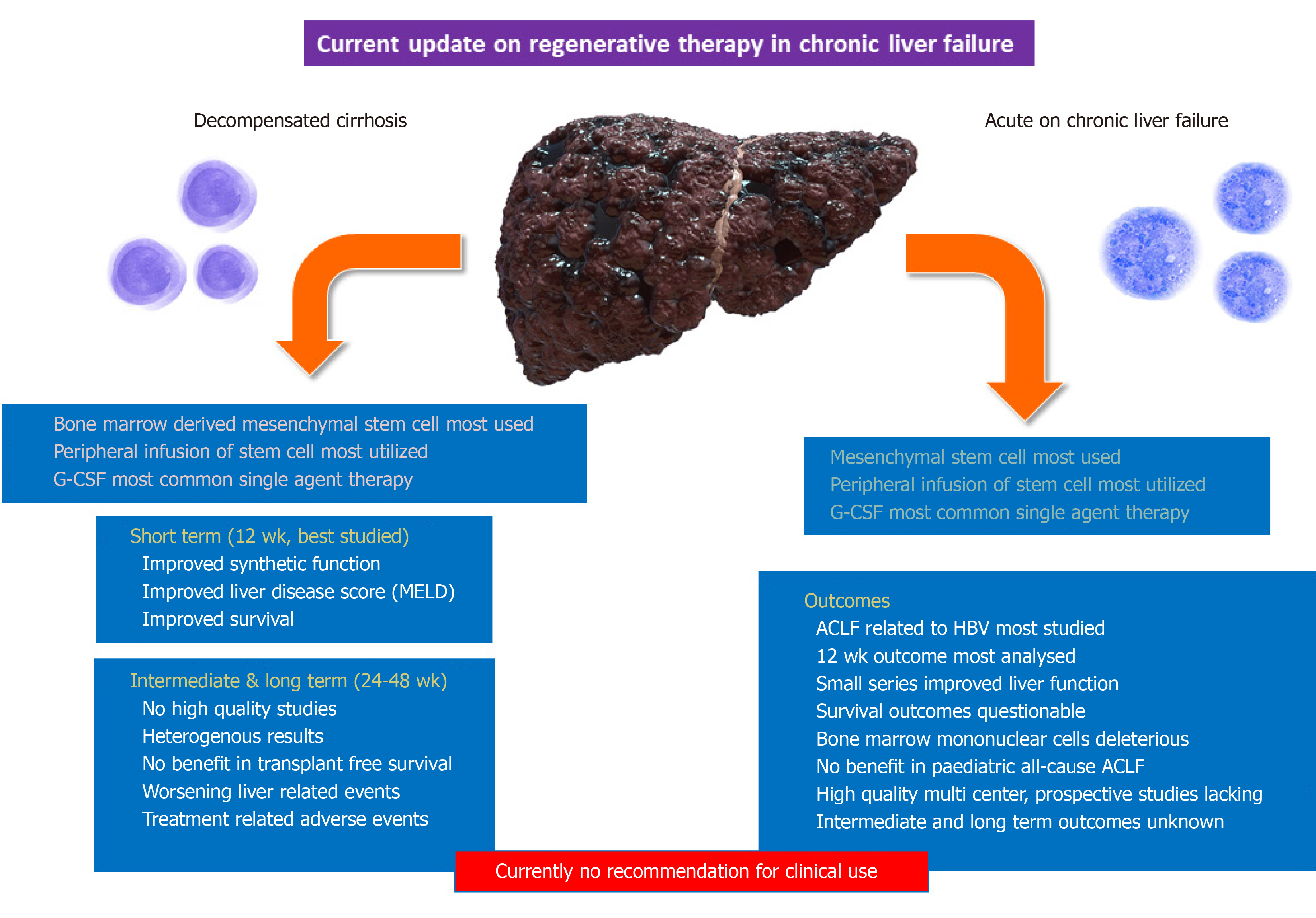
In this regard, a multitude of work has been done on stem cell-based treatment in patients with decompensated cirrhosis. A recent pooled analysis of MSC-based therapy for liver disease inclusive of acute liver failure, chronic liver failure, and ACLF showed that MSC-based therapy was relatively safe and improved liver function during the first six months post treatment. The optimal dose for improvement in liver functions using bone marrow derived MSCs was found to be a single injection via the hepatic artery. Nonetheless, very significant heterogeneity among studies and discontinuous results on sub-group meta-analysis were evident. Thus, the long-term efficacy of MSC therapy was unanswered[17].
In another systematic review on stem cell therapy in chronic liver failure, eight case studies and 17 studies with controls revealed that the most common stem cells were autologous adult stem cells and MSCs from the bone marrow or umbilical cord[18]. These performed better than other types of adult stem cells even though none of the trials reported outcomes in the long-term. The routes of stem cell infusion did not affect the outcomes and fresh or cryopreserved autologous or allogeneic MSCs were equally beneficial. In one study, the use of mononuclear cells derived from the bone marrow resulted in a higher incidence of hepatocellular carcinoma in the long term.
Some quality studies have utilized G-CSF, and cirrhosis patients either developed a worsening model for end stage liver disease (MELD) score, new onset acute kidney injury, thrombocytopenia (due to worsening portal hypertension), or variceal bleeding. The analysis also showed that excessive cell infusion through the portal vein or the hepatic artery route resulted in worse clinical symptoms[18]. Similar findings were noted in a large single center study of decompensated cirrhosis patients awaiting liver transplantation receiving G-CSF in a spaced regimen over one month. Versus a matched historical control group, patients receiving G-CSF had higher mortality (75% vs 46%, P = 0.04) at one year[19]. A well-performed randomized controlled trial on the use of G-CSF with or without haemopoietic stem-cell infusion in chronic liver failure did not improve liver dysfunction or fibrosis and was demonstrably associated with increased frequency of adverse events vs standard care[20]. Even with a large number of studies conducted on stem cell therapy in advanced chronic liver failure, efficient and cost-effective differentiation and production protocols need to be developed, and the safety and true efficacy in the intermediate and long term remain unclear[21]. Studies on the use of stem cell and growth factor therapy in patients with ACLF are less common than those in decompensated cirrhosis. In ACLF patients, apart from mitigation of underlying fibrosis and hepatocyte loss, stem cell therapy aims to ameliorate and modify the systemic and local inflammation toward beneficial inflammatory responses that promote liver regeneration. A narrative review and core summary on ACLF are discussed in the subsequent section.
Zhu et al[22] studied the benefits of porcine adipose-tissue derived stem cells (ADSCs) in a rabbit model of ACLF (chronic liver disease using CCl4 intraperitoneal injection and acute event by intravenous D-galactosamine). The authors found that rodents receiving ADSCs had improved biochemical parameters and histomorphology score of the liver. The authors speculated that ADSCs, like bone marrow derived stromal cells, could differentiate into hepatocytes and promote beneficial inflammatory pathways of regenerative potential. This study however, did not provide evidence for the determined hypothesis. Shi et al[23] and colleagues utilized umbilical-cord derived MSCs (UCMSCs) in patients with ACLF due to hepatitis B virus infection. In this open-label controlled study, with a survival end-point of 72 wk, stem cell therapy was given three times at four-week intervals (two cycles). The authors found that UCMSCs significantly increased the survival of ACLF patients while also reducing liver disease severity scores, improved liver synthetic and excretory functions, and increased platelet counts. Another study on peripheral venous infusion of allogenic bone marrow-derived MSC treatment in ACLF due to hepatitis B infection also demonstrated an increased six-month survival rate associated with improved liver function. There was also decreased incidence of severe infections[24]. These studies described beneficial clinical outcomes with use of different tissue derived stem cells, but did not shed light on the mechanistic actions or provide evidence on the ‘fate-of-infused-cell’ contributing to hepatic progenitor cell repopulation and liver mass reserve. Furthermore, studies on stem cell use in ACLF have utilized a variety of tissue derived cells which compromise conclusive evidence for accurate identification of type of stem cell that is clinically beneficial.
A systematic review on stem MSC transplantation for ACLF due to hepatitis B virus found three studies with 198 patients (91 treated with MSC and 107 on standard medical therapy, controls). One of the studies included in this meta-analysis utilized MSC transplantation along with therapeutic plasma exchange which could have affected true outcome measures. Nonetheless, pooled results showed that MSC treatment could significantly reduce the mortality rate and hyperbilirubinemia at three months and was safe. Nonetheless, this systematic review and its outcomes were confined to short term follow up with intermediate and long-term benefits of MSC transplantation in ACLF due to hepatitis B remain unstudied[25]. In a systematic review and meta-analysis on the clinical performance of stem cell therapy in patients with ACLF, Xue et al[26]and colleagues found beneficial outcomes with stem cell therapy were satisfied in patients with ACLF only in the short-term. They also concluded that MSCs may be better than BMMNCs in the stem cells transplantation of ACLF. This systematic analysis not only included ACLF patients, but also those with acute decompensation and very advanced chronic liver failure patients. Nonetheless, the actual utility and transplant-free survival of ACLF patients on various stem cell-based treatments remain a ‘dwelling possibility’ without real life basic science driven conviction (Figure 2).
Very recently, an interim analysis of the first prospective, randomized, controlled multicenter trial of G-CSF (as a growth factor for bone marrow-stimulated HemSCs) in ACLF was prematurely terminated after conditional power calculation. The researchers found that G-CSF did not show any therapeutic efficacy, did not improve survival, and was associated with adverse events. These findings were in obvious contrast to the results from smaller clinical trials published previously[27]. Similarly, a study of G-CSF in all-cause pediatric ACLF (5 mcg/(kg·d) for 5 d) was found to be ineffective in improving the survival outcome on day 30 and 60 of therapy. The authors called for studies with larger number of children and longer duration of therapy[28]. To summarize (Table 1), peripherally infused mesenchymal stem cells are the most commonly studied regenerative therapy in patients with ACLF with hepatitis B the most common etiology. The other common ‘regenerative’ options include growth factors, especially G-CSF. Even though some studies showed improved short-term survival and reduction in liver dysfunction with MSC-based therapy, strong evidence for recommending stem cell use in ACLF is currently lacking due to the heterogeneity in tissue sources for stem cell derivation and differences in route of administration, dose and duration. Recent studies also caution regarding the use of G-CSF for bone marrow-derived hematopoietic stem cell stimulation in patients with ACLF.
| Ref. | Country | Etiology of ACLF | Type of study | Therapy used | Outcome |
| Shi et al[23], 2012 | China | Hepatitis B virus | Open label, randomized controlled trial (n = 24 treated, 19 controls) | Umbilical cord derived mesenchymal stem cells; cubital vein infusion; 3 times 4 weeks apart | Follow up at 72 wk; partial improvement in MELD score and liver function; 12-wk survival better in stem cell group; no long-term survival benefit |
| Lin et al[24], 2017 | China | Hepatitis B virus | Open label, non-blinded randomized controlled trial (n = 56 treated, 54 controls) | Allogenic bone marrow derived mesenchymal stem cells; peripheral vein infusion; once weekly for 4 wk | Follow up at 24 wk; survival better in stem cell group; bilirubin reduction and MELD score improved significantly in stem cell group |
| Chen et al[25], 2018 | China | Hepatitis B virus | Systematic review and metanalysis of three studies (n = 91 treated with MSC, 107 on SMT) | Bone marrow derived and umbilical cord derived mesenchymal stem cells; one study included patients on plasma exchange along with stem cell therapy (not ideal for inclusion) | Significant reduction of bilirubin at 4 wk and not beyond; improved survival at 12 wk (short term) only; safety profile of peripheral infusion confirmed |
| Xue et al[26], 2018 | China | Multiple | Systematic review and metanalysis of four randomized controlled trials and six non-randomized controlled trials; poor inclusion, decompensated cirrhosis patients, alcoholic cirrhosis and acute liver failure due to hepatitis B virus also included | Bone marrow mononuclear cells, peripheral blood derived stem cells, bone marrow derived stem cells, umbilical cord derived mesenchymal stem cells; peripheral vein and hepatic artery | Significant reduction in bilirubin at 12 mo; improvement in albumin level in long term (confounders not controlled); short term survival at 12 wk; bone marrow mononuclear cells associated with adverse events; no long-term clinical efficacy or safety could be assessed |
| Engelmann et al[27], 2019 | European multicenter | Multiple (mostly alcoholic hepatitis and bacterial sepsis) | Interim analysis of a prospective, controlled, open-label 2-armed study (n = 163) | Granulocyte-colony stimulating factor (5 µg/(kg·d); 12 injections in total, 5 d consecutively, then 3 d apart) | No survival benefits; no clinical improvement; adverse events more with therapy; futility confirmed and study prematurely stopped |
| Sharma et al[28], 2020 | India | Paediatric population (aged > 1 yr); multiple etiology (including Wilsons disease and autoimmune hepatitis) | Open-label randomised pilot study | Granulocyte-colony stimulating factor (5 µg/(kg·d); once daily for 5 d) | Improvement in Child Pugh scores at 2 wk, not thereafter; no clinical improvement in the short-term; no survival benefit at 30-d and 60-d follow up |
Stem cell therapy for patients with ACLF currently remains in the domain of experimental clinical research; strong evidence for its use in routine clinical practice does not exist. This ‘dwelling in the possibility’ for stem cell based regenerative therapy in ACLF has been under study for almost more than half a decade and has not shown any promising value because the dose, duration, timing, route, and type of stem cells with the best efficacy and safety profile in this group of patients remain undefined similar to that in patients with decompensated cirrhosis.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Tanabe S S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
| 1. | Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 2. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 3. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2011] [Cited by in F6Publishing: 2037] [Article Influence: 226.3] [Reference Citation Analysis (2)] |
| 4. | Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, Fernández J, To U, García-Tsao G, Schnabl B. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 5. | Jalan R, Perricone G, Moreau R, Arroyo V, Williams R. Acute-on-chronic liver failure: A new disease or an old one hiding in plain sight? Clin Liver Dis. 2020;15:S45-S51. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Rastogi A, Maiwall R, Bihari C, Trehanpati N, Pamecha V, Sarin SK. Two-tier regenerative response in liver failure in humans. Virchows Arch. 2014;464:565-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Kedarisetty CK, Anand L, Khanam A, Kumar A, Rastogi A, Maiwall R, Sarin SK. Growth factors enhance liver regeneration in acute-on-chronic liver failure. Hepatol Int. 2014;8 Suppl 2:514-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Liu Q. Role of cytokines in the pathophysiology of acute-on-chronic liver failure. Blood Purif. 2009;28:331-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Böhm F, Köhler UA, Speicher T, Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2:294-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Hoffmann K, Nagel AJ, Tanabe K, Fuchs J, Dehlke K, Ghamarnejad O, Lemekhova A, Mehrabi A. Markers of liver regeneration-the role of growth factors and cytokines: a systematic review. BMC Surg. 2020;20:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Tao Y, Wang M, Chen E, Tang H. Liver Regeneration: Analysis of the Main Relevant Signaling Molecules. Mediators Inflamm. 2017;2017:4256352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Xiang X, Feng D, Hwang S, Ren T, Wang X, Trojnar E, Matyas C, Mo R, Shang D, He Y, Seo W, Shah VH, Pacher P, Xie Q, Gao B. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol. 2020;72:736-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 13. | Than NN, Tomlinson CL, Haldar D, King AL, Moore D, Newsome PN. Clinical effectiveness of cell therapies in patients with chronic liver disease and acute-on-chronic liver failure: a systematic review protocol. Syst Rev. 2016;5:100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6:245sr2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | Iansante V, Chandrashekran A, Dhawan A. Cell-based liver therapies: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2018;373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Hu C, Zhao L, Li L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res Ther. 2019;10:199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Zhao L, Chen S, Shi X, Cao H, Li L. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res Ther. 2018;9:72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | AdiwinataPawitan J. Exploring the Most Promising Stem Cell Therapy in Liver Failure: A Systematic Review. Stem Cells Int. 2019;2019:2782548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Philips CA, Augustine P, Rajesh S, Ahamed R, George T, Padsalgi G, Paramaguru R, Valiathan G, John SK. Granulocyte Colony-Stimulating Factor Use in Decompensated Cirrhosis: Lack of Survival Benefit. J Clin Exp Hepatol. 2020;10:124-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Newsome PN, Fox R, King AL, Barton D, Than NN, Moore J, Corbett C, Townsend S, Thomas J, Guo K, Hull D, Beard HA, Thompson J, Atkinson A, Bienek C, McGowan N, Guha N, Campbell J, Hollyman D, Stocken D, Yap C, Forbes SJ. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 21. | Kwak KA, Cho HJ, Yang JY, Park YS. Current Perspectives Regarding Stem Cell-Based Therapy for Liver Cirrhosis. Can J Gastroenterol Hepatol. 2018;2018:4197857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Zhu W, Shi XL, Xiao JQ, Gu GX, Ding YT, Ma ZL. Effects of xenogeneic adipose-derived stem cell transplantation on acute-on-chronic liver failure. Hepatobiliary Pancreat Dis Int. 2013;12:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, He W, Geng H, Jin L, Liu Z, Wang FS. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 24. | Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Weng WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, Gao ZL. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 25. | Chen B, Wang YH, Qian JQ, Wu DB, Chen EQ, Tang H. Human mesenchymal stem cells for hepatitis B virus-related acute-on-chronic liver failure: a systematic review with meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:1224-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Xue R, Meng Q, Dong J, Li J, Yao Q, Zhu Y, Yu H. Clinical performance of stem cell therapy in patients with acute-on-chronic liver failure: a systematic review and meta-analysis. J Transl Med. 2018;16:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Engelmann C, Herber A, Bruns T, Schiefke I, Zipprich A, Schmiedeknecht A, Zeuzem S, Goeser T, Canbay AE, Berg C, Trebicka J, Uschner FE, Mueller T, Aehling N, Krohn S, Schmelzle M, Splith K, Lammert F, Lange CM, Sarrazin C, Trautwein C, Manns MP, Haeussinger D, Pfeiffenberger J, Galle PR, Franke A, Berg T. Granulocyte-Colony Stimulating Factor (G-Csf) to treat acute-on-chronic liver failure (GRAFT Trial): interim analysis of the first randomised European multicentre trial. Hepatology. 2019;70:12A-13A. [Cited in This Article: ] |
| 28. | Sharma S, Lal SB, Sachdeva M, Bhatia A, Varma N. Role of Granulocyte Colony Stimulating Factor on the Short-Term Outcome of Children with Acute on Chronic Liver Failure. J Clin Exp Hepatol. 2020;10:201-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |