Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1184
Peer-review started: February 29, 2020
First decision: April 25, 2020
Revised: May 15, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: October 26, 2020
Liver organoids have recently been applied as models for liver disease and drug screening, especially when combined with liver-on-a-chip technologies. Compared to hepatocyte-like cells, primary hepatocytes have high functionality but cannot maintain their function when cultured in vitro. Mesenchymal stem cells (MSCs) enhance hepatocyte function and maintain hepatocyte metabolism when co-cultured with hepatocytes. MSCs can help induced pluripotent stem cells to generate an organoid structure via the MSC-based traction force triggered by extracellular matrix (ECM) proteins. In this study, primary hepatocytes were co-cultured with MSCs on a liver-derived ECM to generate liver organoids within a short duration.
To create hepatocyte organoids by co-culturing primary hepatocytes with MSCs on a porcine liver extracellular matrix (PLECM) gel.
Perfusion and enzymatic hydrolysis were used to form the PLECM gel. Rat hepatocytes and human MSCs were mixed and plated on pre-solidified PLECM gel in a 48-well plate for 48 h to generate organoids. Generated organoids were evaluated through hematoxylin and eosin, periodic acid-Schiff, immuno-histological, and immunofluorescence staining, and quantitative PCR for alb, CYP450 gene markers, and urea cycle genes. Culture medium was collected to detect albumin (ALB) and urea production on days 2, 4, 6, 8, 14, and 20.
The whole porcine liver was perfused and enzymatically hydrolyzed to form a PLECM gel. The structural components and basement membrane composition of the ECM, such as collagen type I, collagen type IV, fibronectin, and laminin, were demonstrated to be retained. Through interaction of human MSCs with the liver-derived ECM, primary hepatocytes and human MSCs assembled together into a 3D construction and generated primary hepatocyte organoids for 48 h. The mRNAs of the gene alb, the CYP450 gene markers cyp1a1, cyp1a2, and cyp3a2 as well as urea cycle genes arg-1, asl, ass-1, cps-1, nags were highly expressed in hepatocyte organoids. Long-term survival of the primary hepatocyte organoids, as well as stable functionality, was demonstrated via ALB and urea production in vitro.
Our new method of creating primary hepatocyte organoids by co-culturing hepatocytes with MSCs on liver-derived ECM hydrogels could be used to develop models for liver disease and for drug screening.
Core Tip: Mesenchymal stem cells (MSCs) can help primary hepatocytes to create hepatocyte organoids by interacting with a liver-derived extracellular matrix. MSCs and hepatocytes self-assembled together into hepatocyte organoids via MSC-derived condensation related to myosin-II regulatory light chain. The hepatocyte organoids can survive for a long time and maintain the functionality of the hepatocytes while avoiding the limitation of rapid function loss of primary hepatocytes in vitro. This hepatocyte organoid technology can also be used to develop models for liver disease and drug screening.
- Citation: He YT, Zhu XL, Li SF, Zhang BQ, Li Y, Wu Q, Zhang YL, Zhou YY, Li L, Qi YN, Bao J, Bu H. Creating rat hepatocyte organoid as an in vitro model for drug testing. World J Stem Cells 2020; 12(10): 1184-1195
- URL: https://www.wjgnet.com/1948-0210/full/v12/i10/1184.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i10.1184
Organoid technologies have been used recently to build models for disease and drug screening in recent years[1-6], especially when combined with organ-on-a-chip technologies[7,8]. In terms of a model to be used for liver disease and drug screening, fresh primary hepatocytes are the best choice because of their incomparable advantages in functionality and cell characteristics that maintain drug-metabolizing capacity. However, a major challenge that has limited the use of hepatocytes in organoids is the propensity of hepatocytes to lose the ability to replicate and retain liver-specific functions in vitro[9,10]. So far, organoids have been generated with the use of adult stem cells (including progenitor cells)[4,11,12] or pluripotent stem cells[6,13,14]. However, these liver-like cells formed from stem cells are still different from primary hepatocytes with limited liver function and characteristics[10,15]. Therefore, we considered whether there is a way to generate liver organoids from primary hepatocytes within a short duration.
Mesenchymal stem cells (MSCs) can be easily isolated, cultured, and amplified in vitro, and can not only differentiate into a variety of tissue and cell types but also have immunomodulatory effects[16,17]. However, their mesenchymal properties, which are typically used to facilitate cell growth and tissue remodeling, are often underutilized. Previous studies have demonstrated that hepatocytes co-cultured with MSCs enhanced hepatocyte function and maintained hepatocyte metabolism[18,19]. A series of studies by Takebe et al[5,6,13] reported the generation of vascularized and functional liver organoids from various cells including induced pluripotent stem cell (iPSC)-differentiated hepatocytes, human umbilical vein endothelial cells (HUVECs), and MSCs. These studies emphasized that MSCs help in the generation of the organoid structure via a MSC-based traction force triggered by extracellular matrix (ECM) proteins[5]. To our knowledge, this is the first study wherein primary hepatocytes were co-cultured with MSCs to create organoids via interaction with a liver-derived ECM hydrogel and showed long-term survival and stable function in vitro.
Three male Bama miniature pigs weighing 6 kg were purchased from the Animal Experiment Center of Sichuan University (Chengdu, China). Six male Sprague-Dawley (SD) rats were purchased from Dashuo Biotechnology (Chengdu, China). All animals were housed in singular standard cages in an air-conditioned room (21-25 °C), with a 12 h light/dark cycle. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Animal Experiment Center of Sichuan University (IACUC protocol number: [2020007A]). All animals were cared for in accordance with the requirements of the Laboratory Animal Welfare Act and amendments.
Methods used to isolate the porcine liver extracellular matrix (PLECM) have been described previously[20]. The male Bama miniature pigs were anesthetized with Zoletil 50 (10 mg/kg body weight, Virbac, France) and maintained with propofol, and the whole liver was harvested. The liver was infused, in vitro, with Triton X-100 (10 g/L) at a rate of 200 mL/min for 3 h, followed by infusion with deionized water for 3 h. After that, the liver was perfused with 10 g/L sodium dodecyl sulfate (SDS) and then deionized water for 3 h at 200 mL/min. To remove residual SDS, the liver was washed with 36 L of 10 g/L Triton X-100. Subsequently, the liver was washed with 20 L of distilled water to remove residual detergent, followed by PBS for 3 h at 200 mL/min.
For histological examination, the PLECM was fixed in 40 g/L paraformaldehyde, dehydrated, and embedded in paraffin. The PLECM pieces were deparaffinized and stained with hematoxylin and eosin (HE), with 4,6-diamidino-2-phenylindole (DAPI) used for counterstaining. For the immunofluorescence (IF) analysis, slices were embedded in a 50 mg/mL bovine serum albumin (BSA) solution, incubated sequentially for 1 h at room temperature with diluted primary antibodies against collagen type I (cat#ab6308, Abcam, Cambridge, MA, United States), collagen type IV (cat#ab6586, Abcam), fibronectin (cat#ab6328, Abcam), laminin (cat#ab11575, Abcam), followed by incubation with secondary antibodies and DAPI counterstaining. Fresh and PLECM samples (30 mg) were analyzed to quantify DNA content using a commercially available kit (Tiangen Biotech Corporation, Beijing, China) according to the manufacturer’s instructions. PLECM samples were fixed in 25 g/L glutaraldehyde. The samples were dehydrated using a graded ethanol series, dried using the critical point drying method, and then sputter-coated with gold to obtain electrical conductivity. The samples were then observed using a scanning electron microscope.
The PLECM was cut into small pieces and then lyophilized for 48 h. Following lyophilization, samples were ground into a powder with a freezer mill. The PLECM powders were mixed in a solution of 1 mg/mL porcine pepsin (Sigma-Aldrich, St. Louis, MO, United States) in 0.01 mol/L HCl for 48-72 h at room temperature stirring constantly until the ECM powder was fully digested. To form pre-PLECM gels, the pH of the PLECM digest was adjusted to 7.5 using NaOH at 4 °C. The pre-PLECM gel turns into a gel when kept at 37 °C.
SD rats were anesthetized with 45 mg/kg sodium pentobarbital by intraperitoneal injection. Hepatocytes were isolated using a two-step perfusion method as described previously[21]. Freshly isolated hepatocytes were suspended in serum-free medium (SFM). Primary hepatocytes showed a 95% viability when stained with 4 g/mL trypan blue.
Human umbilical cord MSCs (hUC-MSCs) were purchased from Zhongkeweixin Biotechnology (Chengdu, China) and maintained in complete medium (DMEM-LG with 100 mL/L FBS and 100 µg/mL penicillin and 50 µg/mL streptomycin) in an incubator supplied with a humidified atmosphere of 50 mL/L CO2 at 37 °C. For flow cytometric detection of surface antigens, hUC-MSCs were detached from flasks. Then, hUC-MSCs were washed and resuspended at a concentration of 1 × 106 viable cells/mL for 30 min in the dark at 2-8 °C in PBS containing saturation concentrations (1:100 dilution) of the following conjugated mouse or rat monoclonal antibodies against human antigens (BD Biosciences, San Jose, CA, United States): CD34-APC-A, CD45-FITC-A, CD90-PE, CD73-PCA, CD105 PE-A Isotype control, CD19-FITC Isotype control, CD11b-APC Isotype control, and HLA-DR-PC5.5 Isotype control. Cells were washed twice and resuspended in 500 μL of PBS for flow cytometry (FACS Aria, BD Biosciences) using FLOWJO TM software (TreeStar, Inc., Ashland, OR, United States).
To generate a liver organoid in vitro, 7.5 x 105 hepatocytes and 1.5 x 105 MSCs were suspended in a mixture of DMEM-LG and hepatocyte SFM and plated on pre-solidified PLECM gel in a 48-well plate (H+M+E group). After approximately 36-48 h of culture, hepatocytes and MSCs self-organized into organoids. As a control, the same number of mixed hepatocytes and MSCs were seeded in PLECM gel-free plates (H+M group), and the same number of hepatocytes were seeded in PLECM gel-free plates (H group) or PLECM gel pre-coated plates (H+E group). The viability of cells was evaluated using the Fluoroquench fluorescence viability stain (One Lambda, Canoga Park, CA, United States). MSCs and hepatocytes were labeled using the Cell-Tracker Kit (Molecular Probes, Eugene, OR, United States) to identify their location in the 3D organoid. Hepatocytes were labeled green with CellTracker Green CMFDA (C2925), and MSCs were labeled red with CellTracker Red CMTPX (C34552) before seeding. The organoid was visualized using a laser confocal microscope (A1SI, Nikon, Tokyo, Japan).
Western blot analysis was performed to detect the presence of myosin-II regulatory light chain (MRLC) in the PLECM-gel MSC cultures (M+E group), PLECM gel-free MSC cultures (M group), hepatocyte organoids (H+M+E group), and a mixed culture of MSCs and hepatocytes on PLECM gel-free plates (H+M group). The cells were dissolved in RIPA lysis buffer (P0013B, Beyotime, Haimen, China) with PMSF (ST506, Beyotime, Haimen, China). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Tokyo, Japan), which were then probed with the MRLC antibody (cat#ab92721, Abcam). β-actin (cat#ab16039, Abcam, Cambridge) was used as a control for protein loading. Autoradiographs were quantified using Imagelab software.
Organoids were excised from the ECM gel using pipettes, and fixed in 4% paraformaldehyde at room temperature for 3 h. A graded ethanol series was then used to dehydrate the organoids, after which they were immersed in xylene and embedded in paraffin. The organoids were cut into 4 µm sections and then underwent HE, periodic acid-Schiff (PAS), and immunohistological staining. For immunohistological staining, sections were rehydrated, incubated in antigen retrieval solution, and stained using antibody against Ki67 (cat#ab15580, Abcam). For immunofluorescence analysis, sections were embedded in 50 mg/mL BSA solution and then incubated sequentially for 1 h at room temperature with diluted primary antibody against albumin (cat#ab106582, Abcam) and then secondary antibody, followed by DAPI counterstaining.
Total RNA was isolated from the organoids and other culture conditions using Trizol reagent (Sigma-Aldrich) according to the manufacturer’s instructions. The Iscript cDNA synthesis kit (Bio-Rad) was used to synthesize cDNA by reverse transcription from RNA. Quantitative PCR for alb, the CYP450 gene markers cyp1a1, cyp1a2, and cyp3a2, and urea cycle genes arg-1, asl, ass-1, cps-1, and nags was performed with SYBR Premix Ex Taq ( Bio-Rad) in a 20 µL reaction system according to the manufacturer’s instructions. All data were obtained with at least three duplicates. β-actin gene was used as the endogenous internal control, and the results were normalized to the hepatocytes (H group) as ΔCt = Cttarget gene - Ctβ-actin; ΔΔCt = ΔCttreated - ΔCtcontrol, and fold = 2−ΔΔCt. Promoter sequences are shown in Table S1.
Albumin (ALB) concentration was tested using an ELISA kit (Rat Albumin ELISA Quantitation set, E110-125; Bethyl, Montgomery, TX, United States) according to the manufacturer's instructions.
Urea concentration in the culture medium was measured using a quantiChrome urea assay kit (BioAssay Systems, Cambridge, United Kingdom) according to the manufacturer’s instructions.
All statistical analyses were performed with SPSS 23.0 (IBM Corp, Armonk, NY, United States), and organized using GraphPad Prism v8.0 software (GraphPad, La Jolla, CA, United States). At least three parallel experiments were conducted using different samples. Data are presented as the mean ± SE, with at least three duplicates for each data. The t-test and one-way ANOVA were used for comparing differences between groups, and post-hoc tests were performed for multiple comparisons using least significant difference and Dunnett-T3 appropriately based on the test of homogeneity variances (P < 0.05, Dunnett-T3). A two-tailed P value of less than 0.05 was considered statistically significant for all analyses.
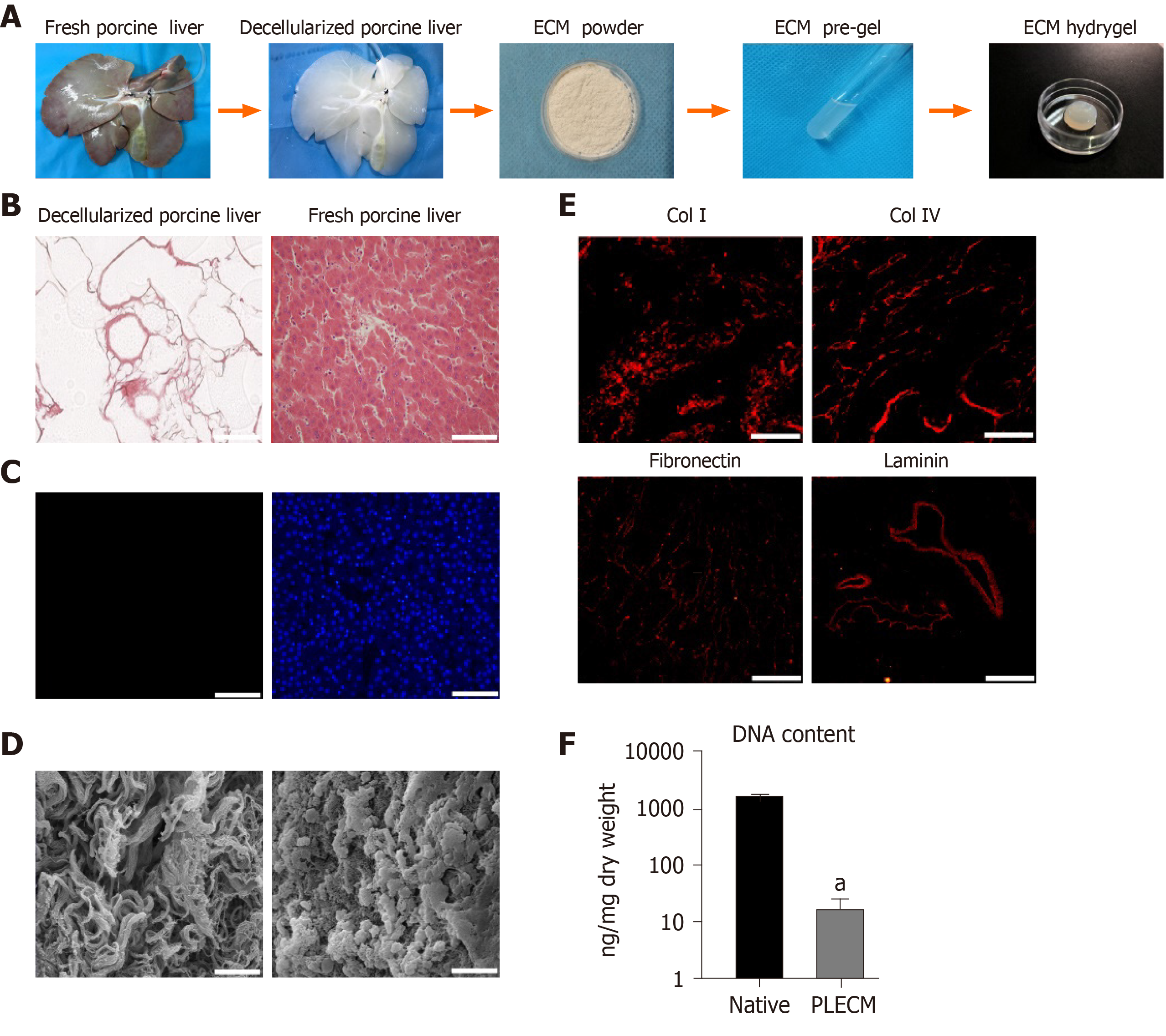
To obtain the PLECM gel, two steps were conducted: Decellularization and solubilization (Figure 1A). The whole liver was perfused from the portal vein using SDS and Triton X-100, which was expected to lead to the following: Destruction of the cellular structure, lysis of the cell membrane, cracking of the nuclear membrane, removal of cell debris, and retention of the collagen and protein structure. After perfusion, the liver became translucent (Figure 1A), and all DNA was removed, as evidenced by the absence of nuclei on both HE (Figure 1B) and DAPI-stained sections (Figure 1C). Scanning electron microscopic images of decellularized liver sections showed that the decellularized liver maintained the ultrastructure of the ECM (Figure 1D). Immunofluorescence test demonstrated that collagen type I, collagen type IV, fibronectin, and laminin, which are structural components, as well as basement membrane composition of the ECM, were retained (Figure 1E). After enzymatic hydrolysis, the ECM formed a transparent semi-gel (Figure 1A). Thus, we obtained an ECM gel with definite elasticity after neutralization. The residual DNA content in the PLECM sections was 1.04% of that in the fresh porcine liver (Figure 1F).
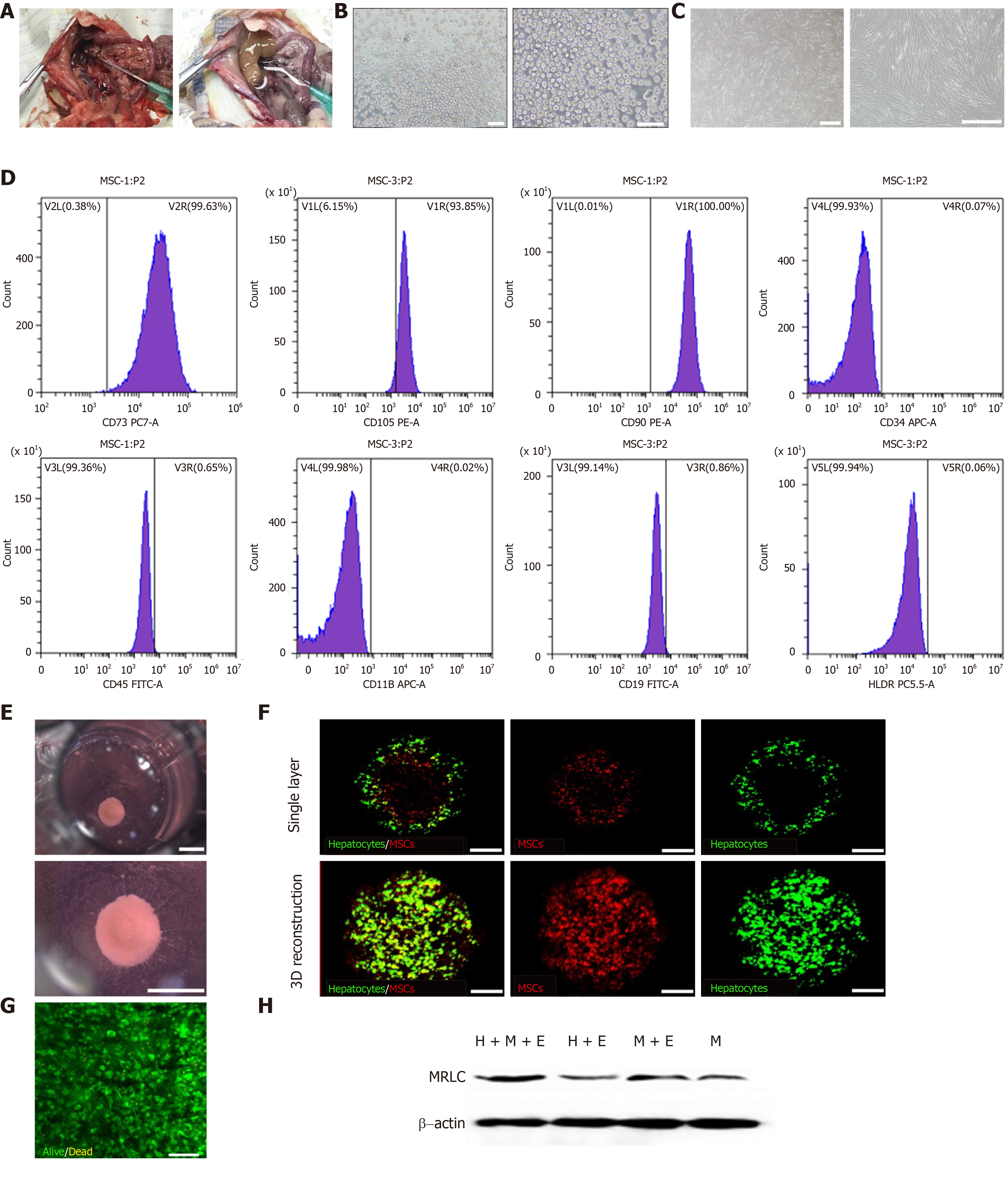
As described previously, hepatocytes were isolated from the portal vein in rat livers by a modified in situ collagenase perfusion technique with a high viability (Figure 2A and B). MSCs showed a homogenous fibroblastic morphology (Figure 2C). Since they were isolated from the human umbilical cord, we identified the cell characteristics by flow cytometry analysis. The cells were positive for the mesenchymal markers (CD73, CD105, and CD90) and negative or low in expression for hematopoietic markers (CD34, CD45, CD19, and CD11b). They also had low immunogenicity and almost no HLA-DR expression (Figure 2D). These results demonstrated that the MSCs were highly pure and expressed the surface marker profile typical of MSCs. Fresh isolated rat primary hepatocytes and P3-P5 MSCs were used in this study. Hepatocytes and MSCs were co-cultured at a ratio of 5:1. The mixed cell lineages on PLECM gel pre-coated plates began to self-aggregate after seeding for 12 h; after 36-48 h, the cells assembled to create organoids and maintained the same size in the culture for 20 d (Figure 2E). 3D reconstruction of the organoid showed that MSCs were scattered around primary hepatocytes (Figure 2F). Most of the primary hepatocytes in organoids were alive at day 2 (Figure 2G). However, the mixed cell lineages did not create organoids under the following conditions: Hepatocytes and MSCs cultured on PLECM gel-free plates, hepatocytes on PLECM gel-free plates, or hepatocytes on PLECM gel pre-coated plates alone. This implied that the lack of MSCs or PLECM gel in the co-culture system resulted in failure to create organoids. As the single MSC cultured on plates showed a homogenous fibroblastic morphology and did not show the capacity for self-condensation, we hypothesized that the co-culture system updated the “contraction mechanism”. Many conventional cellular activities, such as cell adhesion to the ECM and contractile forces produced during cell contraction, involve changes in the cytoskeleton structure[22]. The main protein associated with these changes is non-muscular myosin-II (NSM-II)[23]. We detected the expression of MRLC, which is related to MII activity. MRLC protein expression in PLECM-gel MSC cultures (M+E group) was higher than that in PLECM gel-free MSC cultures (M group), with the same result of higher MRLC protein expression in hepatocyte organoids than in a mixed culture of MSCs and hepatocytes on PLECM gel-free plates (H+M group) (Figure 2H). MSCs that were cultured on PLECM gel pre-coated plates (M+E group) assembled together into some small spheroids. Therefore, we concluded that the substrate matrix contributed to the self-assembly of liver organoids.
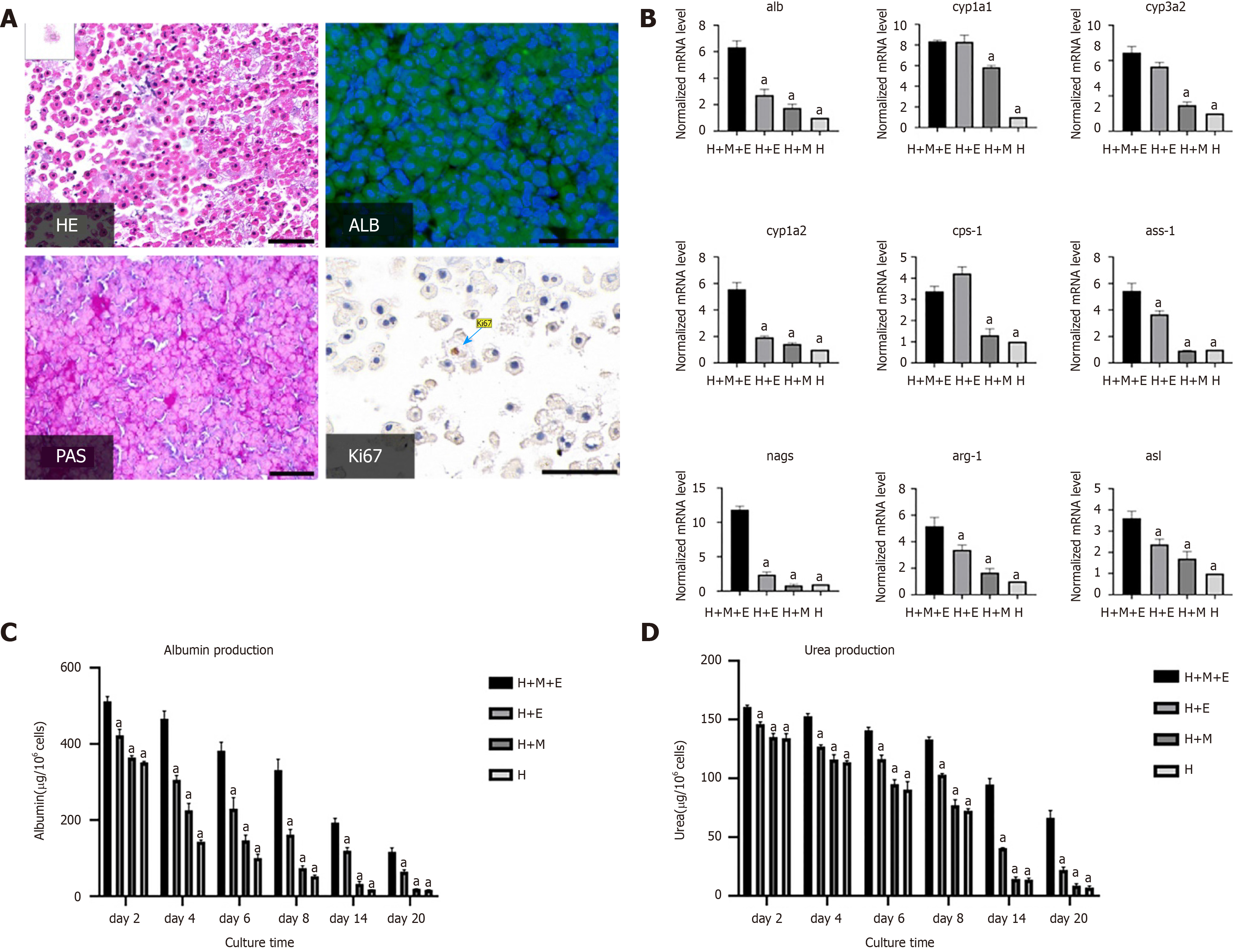
PAS staining was positive and glycogen in the cytoplasm of hepatocytes was stained purple in organoids at day 2 (Figure 3A). Most primary hepatocytes expressed ALB protein as determined by immunofluorescence staining, which indicated that the primary hepatocytes in the liver bud organoids maintained glycogen synthesis and albumin synthesis function after 2 d (Figure 3A). Surprisingly, using immuno-histochemistry, we found that some hepatocytes were proliferating and showed expression of Ki67 (Figure 3A). In addition, organoids at day 2 were harvested for gene expression analysis; the expression levels of the hepatic gene marker alb, CYP450 gene markers cyp1a1, cyp1a2, and cyp3a2, and urea cycle genes arg-1, asl, ass-1, cps-1, and nags increased compared to those in other culture conditions (P < 0.05). These values of organoids were 3.4-11.8 fold higher than those of the hepatocyte group (Figure 3B). We then continued to culture the different groups of cells, described earlier, to determine the duration for which the organoids effectively sustained their function. ALB production and urea detection were used as measures of hepatocyte function and survival time. Culture supernatants were collected on days 2, 4, 6, 8, 14, and 20. On each day, we observed that the production of albumin protein in organoids was significantly higher than that of the other groups (P < 0.05). Without PLECM gel or without MSCs, hepatocytes could not survive for a long time and exhibited rapid loss of function. On day 14, organoids showed significantly higher ALB production (193.31 ± 6.66 µg/106 cells) than the hepatocyte group (18.11 + 0.32 µg/106 cells, P < 0.05) (Figure 3C). Importantly, urea synthesis was improved when primary hepatocytes were co-cultured with MSCs on the PLECM gel to create organoids for up to 3 wk compared with other conditions (Figure 3D). These findings suggested that hepatocyte organoids generated by co-culture with MSCs on the PLECM gel maintained hepatocyte functions and had prolonged survival for at least 20 d.
The use of decellularized tissue PLECM hydrogel can lead to MSC-derived condensation by MRLC to create hepatocyte organoids. The hepatocyte organoids can survive for a long time and maintain their hepatocyte functionality while avoiding the limitation of rapid functional loss of in vitro primary hepatocytes, which are the best cell sources for models to be used for liver disease, drug screening, and cell therapy.
MSC-derived condensation by MRLC and the interplay between cell-hydrogel and cell-cell interactions were essential for the self-assembly of organoids. It was revealed that the MSC-based traction force produced by the actomyosin cytoskeletal axis plays an important role in the directed movements of the liver bud generated from three iPSC-derived lineages on the Matrigel[5]. Furthermore, in Drosophila embryos, gastrulation begins with mesoderm invagination, which is followed by germ-band extension, and both of these processes are morphogenetic movements that are regulated by polarized distribution of myosin II[23]. Similar to the published results, upon using a PLECM hydrogel, our study found that the hepatocyte organoid system updated the expression of MRLC, which is associated with myosin II activity.
PLECM hydrogel provides primary hepatocytes with all necessary information for growth and expansion to generate primary hepatocyte organoids. Regarding the composition of the PLECM, ECM proteins are present in physiologically relevant amounts, and have types I, VI, VII, XI, and XIX collagen, biglycan, and fibronectin, which Matrigel does not have[24]. Matrigel, which is isolated from Engelbreth-Holm-Swarm mouse tumors, has been widely used to generate organoids in many studies rather than a specific decellularized tissue ECM hydrogel[5,11]. In our study, the decellularized liver ECM hydrogel was used to create stable functional organoids. The key advantage of using a decellularized liver ECM hydrogel is the preservation of liver-specific ECM and bioactive molecules, thus providing a microenvironment similar to that of the liver in vivo, which provides the necessary signals for hepatocyte survival and function[25,26].
For generation of organoids, MSCs and rat primary hepatocytes were mixed in a 1:5 ratio with approximately 1 × 106 cells in a 48-well plate. This is a large number of conventional cell cultures within the same base area; in other words, this organoid technique has advantages in function maintenance as well as high cell density culture. However, upon evaluation of cell numbers, we found that a lower number of total cells could not accommodate all cells in a single 48-well together in one organoid, and ended as several smaller ones with scattered distribution within the well. However, a larger number of cells may result in a large organoid, which may lead to poor oxygen supply to cells in the central region. When exploring the cell ratio, we used a series of MSC concentration gradients. We found that a ratio of two cells less than 1:6 resulted in no self-organization into organoids, and increasing the number of MSCs accelerated the generation of organoids (data not shown). Takebe et al[6,13] generated a vascularized and functional human liver by transplantation of liver bud organoids created by human iPSCs in vitro. They used a ratio of 10:2 (iPSCs-hep:MSC/iPSCs-MSC) in 24-well plates or large-scale cultures in one Omni (1: one)-well-array plate[6,13]. Moreover, in a study by Liu et al[18] exploring different co-culture ratios of MSCs and hepatocytes in a scaffold, a co-culture ratio of 1:5 (MSC: Hep) was suggested to be optimal for MSCs to support hepatocellular metabolism and function in vitro, which also could provide better restoration of damaged liver function. Based on these studies, we selected the current ratio of 1:5.
Compared to hepatocyte cultures in sandwich Matrigel, PLECM gel, other hydrogels, or matrices, hepatocyte organoids were similar to the source of the tissue or organ, could show cell-cell interactions and cell-matrix interactions, maintained high quality liver function of primary hepatocytes, and prolonged the survival time of hepatocytes in vitro. Using primary human hepatocytes, such as liver cancer cells, cryopreserved mature human hepatocytes, and human hepatocytes derived from Fah-/-/Rag2-/-/Il2rg-/- mice[27] to generate hepatocyte organoids by our method, hepatocyte organoid technology can also be used in models of liver disease and drug screening for individualized therapy. If this hepatocyte organoid technology is combined with liver-on-a-chip technologies, it can precisely deliver soluble factors to hepatocytes that can maintain prolonged hepatocyte functions in a continuously perfused manner, or even real-time monitoring of cell responses to drug reactions, which can be widely used for multiple tissue engineering applications.
In all cell therapies for liver diseases, primary hepatocyte is accepted as the best cell source with high liver-specific functions compared to immortalized human hepatoblastoma cell lines, nonparenchymal cell, and differentiated cell. But a major challenge that has limited the advancement of these therapies is the propensity of hepatocytes to lose liver-specific functions and the ability to replicate in vitro without microenvironment. Recently, organoid technology has been applied to the study of human development and generation of models for disease and drug screening in different systems such as the brain, stomach, intestine, and kidney. Liver organoids are able to simulate liver development and differentiation, show interaction between cells and cells and between cells and matrix, and can be used in liver transplant, extracorporeal devices, and the generation of models for liver disease and drug screening.
Although many studies reported different liver organoids generated from Lgr5+ stem cells or induced pluripotent stem cell derived hepatocytes liver organoids, few studies have described the generation of liver organoids assembled using mesenchymal stem cells (MSCs) and primary hepatocytes. Besides, almost all studies used Matrigel to create organoids, and we attempted to find a new and reproducible method to generate hepatocytes organoids by co-culture with MSCs on an extracellular matrix (ECM) hydrogel from decellularization porcine liver.
The present study aimed to create hepatocyte organoids by co-culture of hepatocytes with MSCs on a porcine liver extracellular matrix (PLECM) hydrogel.
Porcine liver was infused with Triton and sodium dodecyl sulfate to obtain the porcine liver extracellular matrix (PLECM) scaffold material. After perfusion and enzymatic hydrolysis, PLECM scaffold material formed hydrogel, which was pre-coated in 48 well plate for organoid culture. Rat primary hepatocytes were obtained by two step perfusion method. To create primary hepatocytes organoids, MSCs and rat primary hepatocytes were co-cultured on a PLECM-gel pre-coated plate. HE, PAS, and immunohistological staining, albumin detection, urea production assay, and quantitative PCR for alb, CYP450 gene markers, and urea cycle gene markers were performed to evaluate organoids function.
Decellularized liver maintained the ultrastructure of the extracellular matrix and most important components. MSCs and rat primary hepatocytes generated primary hepatocyte organoids on the PLECM-gel pre-coated plate in 48 h after seeding. MSC condensation on the extracellular matrix hydrogel contributed to the hepatocyte organoids generation. Primary hepatocyte organoids maintained high quality of liver special function of primary hepatocytes and prolonged the survival time of hepatocytes in vitro.
This is the first study to create primary hepatocytes organoids by co-culture of hepatocytes and MSCs on a liver-derived extracellular matrix hydrogel. Hepatocyte organoids show long-term survival and stable function in vitro.
In this study, we used rat hepatocytes to create hepatocytes organoids on a liver-derived extracellular matrix hydrogel. If this method is applied to patients’ liver cancer cells, cryopreserved mature human hepatocytes, and human hepatocytes derived from Fah-/-/Rag2-/-/Il2rg-/- mice, or combined with liver-on-a-chip technologies, it can also be used as models for liver disease and drug screening for individualized therapy.
The authors would like to acknowledge Di-Wei Wu, Qing Yang, and Zhen Yang for their skillful technical assistance.
Manuscript source: Invited manuscript
Specialty type: Engineering, Biomedical
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Murray P S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 838] [Cited by in F6Publishing: 871] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 2. | Ho BX, Pek NMQ, Soh BS. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Li M, Izpisua Belmonte JC. Organoids - Preclinical Models of Human Disease. N Engl J Med. 2019;380:569-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 4. | Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, Korving J, van Boxtel R, Duarte AA, Lelieveld D, van Hoeck A, Ernst RF, Blokzijl F, Nijman IJ, Hoogstraat M, van de Ven M, Egan DA, Zinzalla V, Moll J, Boj SF, Voest EE, Wessels L, van Diest PJ, Rottenberg S, Vries RGJ, Cuppen E, Clevers H. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018; 172: 373-386. e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 784] [Cited by in F6Publishing: 979] [Article Influence: 139.9] [Reference Citation Analysis (0)] |
| 5. | Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, Nakauchi H, Yoshikawa HY, Taniguchi H. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. 2015;16:556-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 315] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, Kitada R, Mori A, Ayabe H, Ejiri Y, Amimoto N, Yamazaki Y, Ogawa S, Ishikawa M, Kiyota Y, Sato Y, Nozawa K, Okamoto S, Ueno Y, Taniguchi H. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017;21:2661-2670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 7. | Rennert K, Steinborn S, Gröger M, Ungerböck B, Jank AM, Ehgartner J, Nietzsche S, Dinger J, Kiehntopf M, Funke H, Peters FT, Lupp A, Gärtner C, Mayr T, Bauer M, Huber O, Mosig AS. A microfluidically perfused three dimensional human liver model. Biomaterials. 2015;71:119-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Au SH, Chamberlain MD, Mahesh S, Sefton MV, Wheeler AR. Hepatic organoids for microfluidic drug screening. Lab Chip. 2014;14:3290-3299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho CS, Choi YJ, Craig Rowlands J, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CE, Gómez-Lechón MJ, Groothuis GM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Häussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhütter HG, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Kevin Park B, Kordes C, Kullak-Ublick GA, LeCluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi J, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EH, Stieger B, Stöber R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM, Hengstler JG. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 1042] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Zhao X, Liang L, Li J, Demirci U, Wang S. A decade of progress in liver regenerative medicine. Biomaterials. 2018;157:161-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 966] [Cited by in F6Publishing: 1023] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 12. | Kuijk EW, Rasmussen S, Blokzijl F, Huch M, Gehart H, Toonen P, Begthel H, Clevers H, Geurts AM, Cuppen E. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci Rep. 2016;6:22154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1350] [Cited by in F6Publishing: 1343] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 14. | Zhang RR, Koido M, Tadokoro T, Ouchi R, Matsuno T, Ueno Y, Sekine K, Takebe T, Taniguchi H. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Reports. 2018;10:780-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Shulman M, Nahmias Y. Long-term culture and coculture of primary rat and human hepatocytes. Methods Mol Biol. 2013;945:287-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Hu C, Li L. The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation. J Transl Med. 2019;17:412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Driscoll J, Patel T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J Gastroenterol. 2019;54:763-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 18. | Liu M, Yang J, Hu W, Zhang S, Wang Y. Superior performance of co-cultured mesenchymal stem cells and hepatocytes in poly(lactic acid-glycolic acid) scaffolds for the treatment of acute liver failure. Biomed Mater. 2016;11:015008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Alzebdeh DA, Matthew HW. Metabolic Oscillations in Co-Cultures of Hepatocytes and Mesenchymal Stem Cells: Effects of Seeding Arrangement and Culture Mixing. J Cell Biochem. 2017;118:3003-3015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Wu Q, Bao J, Zhou YJ, Wang YJ, Du ZG, Shi YJ, Li L, Bu H. Optimizing perfusion-decellularization methods of porcine livers for clinical-scale whole-organ bioengineering. Biomed Res Int. 2015;2015:785474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Bao J, Fisher JE, Lillegard JB, Wang W, Amiot B, Yu Y, Dietz AB, Nahmias Y, Nyberg SL. Serum-free medium and mesenchymal stromal cells enhance functionality and stabilize integrity of rat hepatocyte spheroids. Cell Transplant. 2013;22:299-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165-4178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 23. | Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, Strom SC. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16:1075-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Spang MT, Christman KL. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater. 2018;68:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 26. | Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017;49:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 435] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 27. | Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 600] [Cited by in F6Publishing: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |