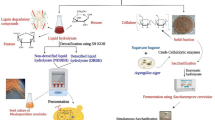
Abstract
The crucial need for alternative oil sources due to increasing energy demand has motivated research to find alternatives resources. In the current study, different fungal groups were isolated from diverse Egyptian localities for oil production screening as well as sugarcane bagasse management. A total of 499 fungal isolates were recovered from soil and dung samples; one isolate was identified as Mortierella wolfii AH12 using morphological and molecular techniques and was demonstrated to produce high lipid concentrations. Taguchi design was used to obtain higher lipid productivity. Accordingly, dry biomass, total lipids, and lipid percentage were 3.81 g l−1, 1.57 g l−1, and 41.2%, respectively, under the optimized culture conditions: 40 g glucose, 5 g peptone, pH 5 at 30 0°C for 10 days. Non-pretreated sugarcane bagasse was not only utilized as an alternative carbon source, but it was also an effective source of nutrients for mold sprouting and lipid biosynthesis using solid-state fermentation (SSF) technique. Promising fatty acid profile was deduced, where palmitic (PA) and oleic acid (OA) were predominant. Moreover, unsaturated fatty acids (USFAs) as linoleic acid (LA), Gamma linolenic acid (GLA), Dihomo gamma linolenic acid (DGLA), arachidonic acid (ARA) were present in low quantities according to GC–MS analysis. The study offers a promising result that serves in the management of agricultural waste, converting it into lipids with a promising profile which could be considered as a precursor for biodiesel.





Similar content being viewed by others
References
Ratledge, C.; Wynn, J.P.: The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 51, 1–52 (2002)
Liang, M.-H.; Jiang, J.-G.: Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog. Lipid Res. 52(4), 395–408 (2013)
Kamyab, H.; et al.: Improved production of lipid contents by cultivating Chlorella pyrenoidosa in heterogeneous organic substrates. Clean Technol. Environ. Policy 21(10), 1969–1978 (2019)
Kamyab, H.; et al.: Evaluation of lipid content in microalgae biomass using palm oil mill effluent (Pome). JOM 69(8), 1361–1367 (2017)
Carota, E.; et al.: Bioconversion of agro-industrial waste into microbial oils by filamentous fungi. Process Saf. Environ. Prot. 117, 143–151 (2018)
Enshaeieh, M.; et al.: Recycling of lignocellulosic waste materials to produce high-value products: single cell oil and xylitol. Int. J. Environ. Sci. Technol. 12(3), 837–846 (2015)
Vivek, N.; et al.: Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate–metabolic aspects, challenges and possibilities: an overview. Bioresour. Technol. 239, 507–517 (2017)
Hashem, A.H., et al.: Eco-green conversion of watermelon peels to single cell oils using a unique oleaginous fungus: Lichtheimia corymbifera AH13. Waste Biomass Valoriz. 1, 5721–5732 (2020)
Suleiman, W.; et al.: Recruitment of Cunninghamella echinulata as an Egyptian isolate to produce unsaturated fatty acids. Res. J. Pharmaceut. Biol. Chem. Sci. 9(1), 764–774 (2018)
Suleiman, W.; et al.: Isolation and screening of promising oleaginous Rhizopus sp and designing of Taguchi method for increasing lipid production. J. Innov. Pharmaceut. Biol. Sci. 5(1), 8–15 (2018)
Wynn, J.P.; et al.: Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi Mucor circinelloides and Mortierella alpina. Microbiology 147(10), 2857–2864 (2001)
Hashem, A.H., et al.: Sustainable lipid production from oleaginous fungus Syncephalastrum racemosum using synthetic and watermelon peel waste media. Bioresour. Technol. Rep. 12, 100569 (2020)
Hoarau, J.; et al.: Sugarcane distillery spent wash, a new resource for third-generation biodiesel production. Water 10(11), 1623 (2018)
Muniraj, I.K.; et al.: Microbial lipid production from potato processing wastewater using oleaginous filamentous fungi Aspergillus oryzae. Water Res. 47(10), 3477–3483 (2013)
Ochsenreither, K.; et al.: Production strategies and applications of microbial single cell oils. Front. Microbiol. 7, 1539 (2016)
Akoh, C.C.: Handbook of Functional Lipids. CRC Press, London (2005)
Gujjala, L.K.; et al.: Biodiesel from oleaginous microbes: opportunities and challenges. Biofuels 10(1), 45–59 (2019)
Enshaeieh, M.; Madani, M.; Ghojavand, S.: Optimizing of lipid production in Cryptococcus heimaeyensis through M32 array of Taguchi design. Process Saf. Environ. Prot. 111, 757–765 (2017)
Madani, M.; Enshaeieh, M.; Abdoli, A.: Single cell oil and its application for biodiesel production. Process Saf. Environ. Prot. 111, 747–756 (2017)
Maji, S., et al.: Agricultural waste: its impact on environment and management approaches. In: Emerging Eco-friendly Green Technologies for Wastewater Treatment. Springer, Berlin, pp. 329–351 (2020)
Avendaño-Morales, B.; Hernández-Martínez, R.; Valdez-Vazquez, I.: Lipid production by Penicillium decumbens from the direct conversion of seaweed bagasse. Rev. Mex. Ingeniería Química 16(3), 691–702 (2017)
Brar, K.; et al.: Potential of oleaginous yeast Trichosporon sp., for conversion of sugarcane bagasse hydrolysate into biodiesel. Bioresour. Technol. 242, 161–168 (2017)
Veana, F.; et al.: Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Braz. J. Microbiol. 45(2), 373–377 (2014)
Cheirsilp, B.; Kitcha, S.: Solid state fermentation by cellulolytic oleaginous fungi for direct conversion of lignocellulosic biomass into lipids: fed-batch and repeated-batch fermentations. Ind. Crops Prod. 66, 73–80 (2015)
Qiao, W.; et al.: Microbial oil production from solid-state fermentation by a newly isolated oleaginous fungus, Mucor circinelloides Q531 from mulberry branches. Royal Soc. Open Sci. 5(11), 180551 (2018)
Gouda, M.K.; Omar, S.H.; Aouad, L.M.: Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J. Microbiol. Biotechnol. 24(9), 1703 (2008)
Olkiewicz, M.; et al.: Evaluation of different sludges from WWTP as a potential source for biodiesel production. Proc. Eng. 42, 634–643 (2012)
Tsegaye, B.; Balomajumder, C.; Roy, P.: Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull. Natl. Res. Centre 43(1), 51 (2019)
Bhatnagar, A.; Kesari, K.K.; Shurpali, N.: Multidisciplinary approaches to handling wastes in sugar industries. Water Air Soil Pollut. 227(1), 11 (2016)
Ahmed, S.U.; et al.: Effects of various process parameters on the production of γ-linolenic acid in submerged fermentation. Food Technol. Biotechnol. 44(2), 283–287 (2006)
Fouda, A.; et al.: Biodegradation and detoxification of bisphenol-A by filamentous fungi screened from nature. J. Adv. Biol. Biotechnol 2, 123–132 (2015)
Lim, S.H.; et al.: Improvement of riboflavin production using mineral support in the culture of Ashbya gossypii. Food Technol. Biotechnol. 41(2), 137–144 (2003)
Mishra, S.K.; et al.: Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Biores. Technol. 155, 330–333 (2014)
El-Sheikh, H.H.; et al.: Impact of some heavy metals on ultrastructure and metabolic profile of Fusarium moniliforme. Afr. J. Mycol. Biotechnol. 19(3), 1–16 (2014)
Khalil, A.M.A.; Hashem, A.H.; Abdelaziz, A.M.: Occurrence of toxigenic Penicillium polonicum in retail green table olives from the Saudi Arabia market. Biocatal. Agric. Biotechnol. 21, 101314 (2019)
Khalil, A.M.A.; Hashem, A.H.: Morphological changes of conidiogenesis in two aspergillus species. J. Pure Appl. Microbiol 12(4), 2041–2049 (2018)
Hasanin, M.S., et al.: Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus microsporus AH3: efficiency and characteristics. Cellulose 27, 4443–4453 (2020)
Kumar, S.; et al.: MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35(6), 1547–1549 (2018)
Folch, J.; Lees, M.; Stanley, G.S.: A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226(1), 497–509 (1957)
Miao, X.; Wu, Q.: Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 97(6), 841–846 (2006)
Hasanin, M.S.; et al.: Isolation and characterization of non-cellulolytic Aspergillus flavus EGYPTA5 exhibiting selective ligninolytic potential. Biocatal. Agric. Biotechnol. 17, 160–167 (2019)
Hasanin, M.S.; Hashem, A.H.: Eco-friendly, economic fungal universal medium from watermelon peel waste. J. Microbiol. Methods 168, 105802 (2020)
Hatfield, R.D.; et al.: A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J. Sci. Food Agric. 65(1), 51–58 (1994)
Cheng, K.-K.; et al.: Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochem. Eng. J. 38(1), 105–109 (2008)
Gupta, A.; et al.: Omega-3 fatty acid production from enzyme saccharified hemp hydrolysate using a novel marine thraustochytrid strain. Bioresour. Technol. 184, 373–378 (2015)
Papanikolaou, S.; et al.: Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenerg. 32(1), 60–71 (2008)
Aguiar, M.M.; Ferreira, L.F.R.; Monteiro, R.T.R.: Use of vinasse and sugarcane bagasse for the production of enzymes by lignocellulolytic fungi. Braz. Arch. Biol. Technol. 53(5), 1245–1254 (2010)
Anwar, S.: Determination of moisture content of bagasse of jaggery unit using microwave oven. J. Eng. Sci. Technol. 5(4), 472–478 (2010)
Fakas, S.; et al.: Fatty acid composition in lipid fractions lengthwise the mycelium of Mortierella isabellina and lipid production by solid state fermentation. Bioresour. Technol. 100(23), 6118–6120 (2009)
Fakas, S.; et al.: Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenerg. 33(4), 573–580 (2009)
Lu, H.; Zhang, B.-B.; Wu, Z.-H.: Studies on mucor racemosus fermentation to manufacture Gamma-linolenic acid functional food douchi. Food Sci. Technol. Res. 16(6), 543–548 (2010)
Zhang, Y.; Adams, I.P.; Ratledge, C.: Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 25-fold increase in lipid accumulation. Microbiology 153(7), 2013–2025 (2007)
Patel, A.; et al.: Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 77, 604–616 (2017)
Benny, G.L.: Methods used by Dr. RK Benjamin, and other mycologists, to isolate zygomycetes. J. Syst. Evolut. Bot. 26(1), 37–61 (2008)
Dyal, S.D.; Narine, S.S.: Implications for the use of Mortierella fungi in the industrial production of essential fatty acids. Food Res. Int. 38(4), 445–467 (2005)
Aki, T.; et al.: Production of arachidonic acid by filamentous fungus, Mortierella alliacea strain YN-15. J. Am. Oil Chem. Soc. 78(6), 599–604 (2001)
Kikukawa, H.; et al.: Arachidonic acid production by the oleaginous fungus Mortierella alpina 1S-4: a review. J. Adv. Res. 11, 15–22 (2018)
Gardeli, C.; et al.: Lipid production and characterization by Mortierella (Umbelopsis) isabellina cultivated on lignocellulosic sugars. J. Appl. Microbiol. 123(6), 1461–1477 (2017)
Diwan, B.; Parkhey, P.; Gupta, P.: From agro-industrial wastes to single cell oils: a step towards prospective biorefinery. Folia Microbiol. 63(5), 547–568 (2018)
Ge, C.; et al.: Application of a ω-3 desaturase with an arachidonic acid preference to eicosapentaenoic acid production in Mortierella alpina. Front. Bioeng. Biotechnol. 5, 89 (2018)
Dzurendova, S., et al.: The influence of phosphorus source and the nature of nitrogen substrate on the biomass production and lipid accumulation in oleaginous Mucoromycota fungi. Appl. Microbiol. Biotechnol. 104, 8065–8076 (2020)
Enshaeieh, M.; Nahvi, I.; Madani, M.: Improving microbial oil production with standard and native oleaginous yeasts by using Taguchi design. Int. J. Environ. Sci. Technol. 11(3), 597–604 (2014)
Chiranjeevi, P.; Venkata Mohan, S.: Optimizing the critical factors for lipid productivity during stress phased heterotrophic microalgae cultivation. Front. Energy Res. 4, 26 (2016)
Nisha, A.; Venkateswaran, G.: Effect of culture variables on mycelial arachidonic acid production by Mortierella alpina. Food Bioprocess Technol. 4(2), 232–240 (2011)
Papanikolaou, S.; et al.: Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. Eur. J. Lipid Sci. Technol. 109(11), 1060–1070 (2007)
Rocky-Salimi, K.; Hamidi-Esfahani, Z.; Abbasi, S.: Statistical optimization of arachidonic acid production by Mortierella alpina CBS 754.68 in submerged fermentation. Iran. J. Biotechnol. 9(2), 87–93 (2011)
Nasr, M.M.; et al.: The effect of carbon and nitrogen sources on the fatty acids profile of Mortierella vinacea. Biol. J. Microorg. 5(20), 1–8 (2017)
Vadivelan, G.; Venkateswaran, G.: Production and enhancement of omega-3 fatty acid from Mortierella alpina CFR-GV15: its food and therapeutic application. BioMed Res. Int. (2014). https://doi.org/10.1155/2014/657414
Kosa, G.; et al.: High-throughput screening of Mucoromycota fungi for production of low-and high-value lipids. Biotechnol. Biofuels 11(1), 66 (2018)
Tang, X.; et al.: Comparison of biochemical activities between high and low lipid-producing strains of Mucor circinelloides: an explanation for the high oleaginicity of strain WJ11. PLoS ONE 10(6), e0128396 (2015)
Broughton, R.: Omega 3 Fatty Acids: Identification of Novel Fungal and Chromistal Sources. University of London, London (2011)
Cohen, Z.; Vonshak, A.; Richmond, A.: Fatty acid composition of Spirulina strains grown under various environmental conditions. Phytochemistry 26(8), 2255–2258 (1987)
Silveira, C.M.d.; Oliveira, M.d.S.; Furlong, E.B.: Conteúdo lipídico e perfil em ácidos graxos de farelos submetidos à fermentação por Aspergillus oryzae em estado Sólido (2010)
Guilherme, A.; et al.: Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Braz. J. Chem. Eng. 32(1), 23–33 (2015)
Zhang, J.; Hu, B.: Solid-state fermentation of Mortierella isabellina for lipid production from soybean hull. Appl. Biochem. Biotechnol. 166(4), 1034–1046 (2012)
Chen, X.; et al.: Improved xylan hydrolysis of corn stover by deacetylation with high solids dilute acid pretreatment. Ind. Eng. Chem. Res. 51(1), 70–76 (2012)
Sindhu, R.; et al.: Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresour. Technol. 102(23), 10915–10921 (2011)
Agbor, V.B.; et al.: Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 29(6), 675–685 (2011)
Singh, D.; Chen, S.: The white-rot fungus Phanerochaete chrysosporium: conditions for the production of lignin-degrading enzymes. Appl. Microbiol. Biotechnol. 81(3), 399–417 (2008)
Soares, I.; et al.: Evaluation of the effects of operational parameters in the pretreatment of sugarcane bagasse with diluted sulfuric acid using analysis of variance. Chem. Eng. Commun. 204(12), 1369–1390 (2017)
Timung, R., et al.: Effect of subsequent dilute acid and enzymatic hydrolysis on reducing sugar production from sugarcane bagasse and spent citronella biomass. J. Energy (2016). https://doi.org/10.1155/2016/8506214
Dussan, K.J., et al.: Dilute-acid hydrolysis of cellulose to glucose from sugarcane bagasse. Chem. Eng. Trans. 38, 433–438 (2014)
Kucharska, K.; et al.: Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 23(11), 2937 (2018)
Vats, S., et al.: Development of a microbial consortium for production of blend of enzymes for hydrolysis of agricultural wastes into sugars (2013)
Mbaneme-Smith, V.; Chinn, M.S.: Consolidated bioprocessing for biofuel production: recent advances. Energy Emission Control Technol. 3, 23 (2015)
Kiatkittipong, W.; Wongsuchoto, P.; Pavasant, P.: Life cycle assessment of bagasse waste management options. Waste Manag. 29(5), 1628–1633 (2009)
Ahmad, F.B., et al.: Microbial oil production from sugarcane bagasse hydrolysates by oleaginous yeast and filamentous fungi. In: Proceedings of the 38th Annual Conference of the Australian Society of Sugar Cane Technologists. 2016. Australian Society of Sugar Cane Technologists-ASSCT.
Ruan, Z., et al.: Oleaginous fungal lipid fermentation on combined acid-and alkali-pretreated corn stover hydrolysate for advanced biofuel production. Bioresour. Technol. 163, 12–17 (2014)
Sorokina, K., et al.: New methods for the one-pot processing of polysaccharide components (cellulose and hemicelluloses) of lignocellulose biomass into valuable products. Part 2: biotechnological approaches to the conversion of polysaccharides and monosaccharides into the valuable industrial chemicals. Catal. Ind. 9(3), 264–269 (2017)
Bajpai, P.K.; Bajpai, P.; Ward, O.P.: Production of arachidonic acid by Mortierella alpina ATCC 32222. J. Ind. Microbiol. 8(3), 179–185 (1991)
Puri, P.; et al.: A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46(4), 1081–1090 (2007)
Acknowledgements
All authors would like to thank the faculty of Science, Al-Azhar University as well as the City for Scientific Research and Technology Applications in which the work steps were done.
Funding
All expenses whether chemical analysis or molecular identification, etc., were funded by ourselves, and there is no funder or funding agency support us to finish this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the same percentage in all stages of the research.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hashem, A.H., Suleiman, W.B., Abu-Elrish, G.M. et al. Consolidated Bioprocessing of Sugarcane Bagasse to Microbial Oil by Newly Isolated Oleaginous Fungus: Mortierella wolfii. Arab J Sci Eng 46, 199–211 (2021). https://doi.org/10.1007/s13369-020-05076-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05076-3