Abstract
Internal friction combined with SEM, DSC and XRD measurements was first applied so far to investigate phase transformation behaviors of Fe-Al elemental powders. Special attentions are concentrated on the influence of mechanical ball-milling on microstructure transition during sintering process. A tailored solid solution degree of the Fe-Al powder mixture can be obtained by mechanical ball-milling for different time. Four typical internal friction peaks are observed as termed P1, P2, P3 and P4 peak respectively for Fe-Al ball-milled powder compact. While for no ball-milled one, only P2 and P4 peaks appear. It was rationalized that the four internal friction peaks are probably associated with the formation of FeAl3, Fe2Al5, FeAl2 and FeAl respectively because of solid diffusion reaction during sintering process. In addition, increasing ball-milling time results in enhancement of solid solution degree owing to refined powder particle and substructure as well as increased solid defect density and interfacial area, and thus phase transformation and corresponding internal friction peaks shift to a lower temperature. The absence of two internal friction peaks in no ball-milled one is ascribed to lower solid solution degree.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Fe-Al intermetallics have attracted considerable interests from practical point of view because of their attractive physical and mechanical properties, especially oxidation and corrosion resistance at higher temperature. In addition, their low cost of raw materials used for their manufacture is also very competitive [1–3]. Many material-processing methods of the Fe-Al intermetallics have been developed to satisfy various object demands, among which powder metallurgy technique accompanying self-propagating high-temperature synthesis (SHS) sometimes may be only a reasonable choice to form precision, high performance products of Fe-Al system owing to lower processing temperture and flexibility in composition and microstructure control [4, 5]. In order to obtain a desirable sintering performance of elemental Fe-Al powder mixture, the powder mixture was usually conducted a ball-milling process, which not only can refine the crystalline grain but also can promote solid solution degree by reducing the activation enengy and generating thermal energy during this process [6, 7].
For many years, an important subject of interest for Fe-Al powder system is focused on systematic investigation of phase formation and transformation behaviors during sintering process owing to the reasons that detailedly understanding the phase transformation is faviorable to adjust sintering procedure and tailor microstructure of final product to meet special requirements. Sina studied reactive synthesis of Fe-40 at.% Al powder mixture in light of DSC, SEM and XRD. Two exothermal peaks were respectively observed corresponding to two stages consisted of precombustion reaction and combustion process for compacted sample containing coarse Fe particles, but compared to relative fine Fe powder, only a single and sharp exothermic peak was found during combustion process. The formation of the Fe2Al5 was considered to account for the appearance of the exothermic peak [8]. Gedevanishvili studied expansion behavior of elemental Fe-Al powder mixture with the chemical composition of Fe-40 at.% Al by dilatometric experiments combined with DSC testing and SEM observation. It was found that dramatic expansion behavior took place associated with the formation of Fe2Al5 corresponding to the appearance of first exothermic peak at around 560 °C, but no obvious thermal expansion took place corresponding to second exothermic peak at around 655 °C which connects the formation of FeAl [9]. According to Fe-Al binary phase equilibrium diagram, the sequence of intermetallic compounds appearing during sintering process should be FeAl3, Fe2Al5, FeAl2 and FeAl [10]. The phase analysis mentioned above didn't indicate the appearance of FeAl3 and FeAl2 except for Fe2Al5 and FeAl formed during sintering process for the elemental Fe-Al powder mixture. Pocheć and Jóźwiak et al investigated the phenomena preceding and accompanying SHS reaction between Fe and Al elemental powder during isothermal sintering based on SEM, DSC and XRD analysis. Their experimantal results demonstrated that in addition to the well-known Fe2Al5 and low Al solid solution of Fe, the rich Al phase FeAl3 and FeAl2 are also observed just before SHS reaction. The Maps of Fe-Al phase formation kinetics parameters were described using JMA (Johnson-Mehl-Avrami), and the activation energy of particular phase formation was determined by the Kissinger method. Moreover, computer analysis of DSC curves allowed splitting of the complex experimental peaks into individual peaks in accordance with the formation of particular phase, i.e. FeAl3, Fe2Al5, FeAl2 and FeAl, indicated that the four successive phases can be formed during sintering process for the elemental Fe-Al powder mixture [11–13].
From the experimental results reported as above references, it can be noticed that phase transition products during sintering process are not completely consistent for the Fe-Al elemental powder mixture, two kinds of intermetallic compounds or four ones appeared. The contradiction is probably ascribled to the difference of powder particle size, raw materials purity, heating rate, sintering environment etc. In addition, constrained the high speed and instantaneity of the SHS reaction and its strongly exothermic nature, it is still a challenge to accurately track dynamic phase transformation process. Continuously exploring new technical methods to clarify Fe-Al dynamic phase transformation evolution law is still necessary as yet.
It well known that mechanical vibration of an object will be gradulally attenuated even if the object is completely isolated from outside. The energy dissipation from mechanical energy to thermal energy is designated internal friction which is a very sensitive technique to track dynamic microstructure evolution [14]. The internal friction spectrum can provide expected informations such as solid defect evolutions even at an atom scale. Moreover, basing on the analysis of typical internal friction phenomina, the movement, distribution and interaction rule of the microdefects can be disclosed [15]. In our past works, two internal friction peaks were found for no ball-milled Fe-Al powder compact during sintering process, and the appearance of the two peaks is rationalized to originate from the formation of Fe2Al5 and FeAl respectively. It also elucidated that the internal friction is also a new candidate to understand phase transformation process [16]. In the present paper, the internal friction technique combined with SEM, DSC, and XRD was adopted to further investigate phase transformation process of Fe-Al powders compact, and special attention are concerntrated on the influence of mechanical ball-milling on phase transformation process.
2. Experimental detail
Elemental Fe ( Alfa, 6–10 μm, 99.5% purity) and Al (Macklin, 25 μm, 99.9% purity) powder particles were prepared as start materials. Powder masses of Fe and Al were firstly calculated and weighed according to the chemical composition of Fe-43 at.% Al. Next, the powder mixture was placed inside a stainless steel vial (250 ml volume) and blended by a planetary high-energy ball mill (Germany, Fritsch-Pulverisette 6) at a rotation speed 200 rpm without grinding ball for 2 h until a uniform distribution was achieved. Then, the mechanical ball-milling was carried out for the blended Fe-Al powder mixture at a rotation speed 150 rpm with the ball/powder weight ration 10:1 for 1 h and 5 h separately to tailor solid solution degree of the mixture. In order to avoid introducing impurities and sample oxidation, ball-milling process was conducted under a vacuum condition of 10–3 Pa and no process control agent was used. Finally, powder mixture after ball-milling was taken out with the help of a glove box unde an argon atmosphere.
Uniaxial compression using table-type powder tablet machine (FYD-40) was conducted to prepare internal friction testing sample with the rectangle dimensions of 65 × 5 × 1.2 mm3 under the pressure of 400 MPa. Internal friction (IF) and relative dynamic modulus (RDM) of Fe-Al power compact during sintering process were investigated using a multifunction intenal friction apparatus (MFIFA-I) initially invented by Kê [15]. The internal friction measurement was performed ranging from 300 to 700 °C under a vacuum atmosphere of 10–3 Pa with the strain amplitude 20 × 10−6 and measuring frequencies 0.5, 1.0, 2.0 and 4.0 Hz. Heating rate is employed 1 °C min−1 unless otherwise specified. The apparatus is mainly consisted of an inverted torsion pendulum, a temperature programmer, a photoelectron transformer and an automatic computer. The computer controls the system to operate the whole measurement and processes data in real time. The detailed operating principle of the apparatus can be found in [17].
Scanning electron microscopy (SEM) analysis was performed on a Hirox SH-4000M to characterize the micro morphology of Fe-Al powder mixture, operated at 30 kV. X-ray diffraction (XRD) measurements were carried out on a Shimaozu XRD-7000. XRD patterns were obtained using Cu Kα radiation for phase identification with a step size of 0.02° and a step time of 0.3 s. Thermal measurements from 300 °C–700 °C were performed using a computer-assisted Netzsch STA449F3 differential scanning calorimeter (DSC) in the specific heat mode at a constant heating rate of 5 °C min−1 with the expection to determine the nature of the reactions (endothermic or exothermic) and the temperature of onset of the various reactions.
3. Results and discussion
The SEM photomicrographs under a backscattered electron mode of the Fe-Al powder mixture undergone various ball-milling time are presented in figure 1. These images distinctly exhibit the microstructure evolution process. It can be observed that a uniform distribution was achieved after no ball-blending for 2 h as shown in figure 1(a). The Al particle exhibits dark grey in color while the Fe particle is white. Figures 1(b) and (c) respectively show the microstructure of ball-milled powder mixture after ball-milling for 1 h and 5 h. It can be found that a strong plastic deformation took place due to impact collision and rolling action of grinding ball. The original spherical particles transit into flat lamellar structure after 1 h ball-milling. At this stage, there is still no microsandwich formation and the two populations of particles of pure Fe and pure Al can be distincted. Increasing ball-milling time up to 5 h results in small and slightly angular particles and decreases particle size. Nearly spherical particles are brought out with sandwich structure. It is difficult to distinguish Fe and Al particle as separate at this stage, indicated that the layer thickness of sandwich structure particle has been greately decreased. This kind of extremely thin layer strcture is very advantage to mutual diffusion of atoms and solid-state reaction. The XRD patterns corresponding to different ball-milling time are displayed in figure 2. It can be noted that the product of ball-milling under present condition is still pure Fe and pure Al as well as Fe-Al solid solution. No any Fe-Al intermetallic compounds were formed even ball-milling time for 5 h. However, it is easily understood that the solid solution degree of the Fe-Al powder mixture can be greatly increased with prolonging ball-milling time due to refined partice size and decreased order degree of crystal structure [18]. The decreased diffraction intensity of XRD is associated with the higher diffusion coefficient of Al than Fe atoms and grains refined.
Figure 1. SEM photomicrographs of Fe-Al powder mixture after ball-milling for (a) 0 h, (b) 1 h, (c), 5 h.
Download figure:
Standard image High-resolution imageFigure 2. XRD patterns of the mixed powder experienced different ball-milling time.
Download figure:
Standard image High-resolution imageFigure 3 shows the typical IF and RDM characteristics as a function of temperature during heating process for the Fe-Al powder compact after 1 h ball-milling at the frequency of 1 Hz. It can be found that the most outstanding features are the appearance of four IF peaks, separately located at around 431 °C, 529 °C, 570 °C and 646 °C. These four IF peaks were respectively termed as P1, P2, P3 and P4 peak. In accordance with the appearance of the IF four peaks, the RDMs exhibit a local minimum, especially corresponding to P2 and P4 peak locations. Figure 4 reflects the evolution process of four IF peaks with respect to ball-milling time. It can be noted that ball-milling time plays a dominant influence on the IF charactersitics. Four IF peaks exhibit noticeable measuring frequency dependence as the peak height greatly decreases with increasing measuring frequency but the peak temperatures are almost constant, clearly indicating phase transformation IF peak features. For the no ball-milling powder compact, only two obvious IF peaks are found corresponding to P2 and P4 IF peaks. As a comparison, four IF peaks were observed with regard to ball-milled powder compact as subjected to ball-milling for 1 h or 5 h. However, increasing ball-milling time up to 5 h, P2, P3 and P4 peaks become less clear as compared to P1 peak which turns more steep and noticeable. Furthermore, it also can be noticed that P1 and P2 peaks shift towards lower temperature when increasing ball-milling time, but P3 and P4 peak locations are seemingly independent of ball-milling time.
Figure 3. IF and RDM as a function of temperature for the Fe-Al power compact ball milled for 1 h.
Download figure:
Standard image High-resolution imageFigure 4. IF properties of Fe-Al power compact processing various ball-milling time.
Download figure:
Standard image High-resolution imageThe formation mechanisms of P2 and P4 IF peaks appearing in the no ball-milling sample have been discussed [16]. It has been concluded that the appearances of the two IF peaks are respectively associated with the formation of Fe2Al5 and FeAl intermetallic compounds during sintering process. The conclusion was further confirmed by the DSC result of figure 5(a) that displays two exothermal peaks appearance as have been reported in [9]. The two IF peaks are involved in two reactions as 2Fe + 5Al = Fe2Al5 and Fe2Al5 + Fe = 5FeAl. Furthermore, the phases of Fe2Al5 and FeAl were observed at the SHS stage related to the combustion reaction by EDS analysis in the work of Sina [11]. Naoi studied the kinetics of the reactive diffusion in the binary Fe-Al system at solid state temperatures using sandwich Al/Fe/Al diffusion couples. The Fe2Al5 layer was also observed on the Fe/Al interface after isothermally annealing [19]. From another point of view, it can be indicated that the IF spectrum is a new candidate to track phase transformation process of Fe-Al power mixture except for traditional technique methods.
Figure 5. DSC diagrams of Fe-Al powder mixture ball milled for various time.
Download figure:
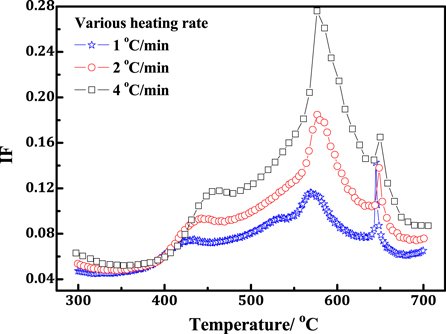
Standard image High-resolution imageAccording to binary Fe-Al equilibrium diagram, the sequence of the phases appearing related to different temperature should be FeAl3, Fe2Al5, FeAl2 and FeAl [10–13]. The activational energy of each phase is different associated with different thermodynamic properties. The P1 peak shifts towards lower temperature as increasing ball-milling time, which locates at around 431 °C when ball-milling time is 1 h. while when ball-milling time increases up to 5 h, the peak temperature reduces to about 401 °C. The DSC results of the ball-milled samples for 1 h and 5 h are respectively exhibited in figures 5(b) and (c). It can be noted that the first exothermic peak appears at around 445 and 425 °C respectively for ball-milled samples for 1 h and 5 h. The P1 peak and the first exothermal peak have similar temperature range and changing trend, indicated identical physical mechanism. The slightly difference in temperature locations for the IF peak and DSC peak is probably ascribed to the difference of heating rate as 1 °C min−1 for IF and 5 °C min−1 for DSC as has been documented in [20]. The dependence of heating rate on IF is shown in figure 6 for the ball-milled 1 h sample. It can be noted that the P1 and P3 peaks obviously shift towards higher temperature when increasing heating rate. However, P2 peak becomes not conspicuous when heating rate is raised up to 2 °C min−1 or 4 °C min−1. The IF peaks appeared at higher heating rate are highly consistant with the appearance of exothermic peaks in figures 5(b) and (c). High heating rate may lead to inadequate solid phase diffusion reaction or coexistence of partial metastable phases, such as FeAl3 + Fe2Al5, Fe2Al5 + FeAl2 owing to shorten time accumulation at solid diffusion stage during sintering process [3, 13], which is probably the reason to account for the inconspicuousness of P2 corresponding to heating rate. Pocheć has confirmed the existence of FeAl3 phase microzone according to SEM observation and chemical composition EDS calibration [11]. The solid state diffusion reaction at the contacts between Fe and Al particles after isothermal sintering at 400 °C or at 450 °C is considered to account for the formation of FeAl3 [11, 12]. Charlot has proven that the ignition temperature of the Fe-40 wt.% Al elemental powder mixture is approximately equal to 400 °C for the mechanically activated sample (200 rpm, 4 h) which is much lower that 500 °C for the sample without a mechanical activation step. In addition, such a result can be observed whatever the Fe/Al compositions [21]. The ignition temperature for the ball-milled sample is much consistent with the P1 IF peak location, indicated the formation of the FeAl3 phase. A minor FeAl3 layer except for Fe2Al5 was also observed by EBSD phase distribution diagram when mild steel was hot dipped in a pure aluminum molten bath [22]. Similar results were also reported by Shahverdi when molten Aluinum comes in contact with solid iron [23]. From the analysis mentioned above, it can be rationalized that the appearance of the P1 peak is probably originated from the formation of FeAl3.
Figure 6. Dependence of IF characteristics on heating rate for the Fe-Al elemental powders ball milled for 1 h.
Download figure:
Standard image High-resolution imageThe FeAl2 phase has been identified by XRD diffraction and SEM observation for Fe-50 at.% Al sample after sintering at 570 °C [11, 12]. In addition, DSC curves being separated into peaks by computer analysis for Fe-Al powder mixture after isothermal annealing at 630 °C is also indicated the creation of FeAl2 [11]. Similar experimental results were obtained by Cardellini that confirmed the appearance of FeAl2 [24]. Gao found that the FeAl2 can coexist with other Fe-Al intermetallic compounds at a certain temperature corresponding to different chemical composition, such as FeAl2 + FeAl, FeAl2 + Fe2Al5 for Fe-Al elemental powders [25]. Furthermore, the P3 peak locating at 570 °C with end temperature about 635 °C is well matched to the exothermal peak appeared in figure 5. As a consequence, it can be concluded that the P2 peak is associated with the formation of FeAl2.
Shifting towards lower temperture of the first exothermic peak and P1 peak can be undstood in light of the increase of solid solution degree resulted from increased ball-milling time. As well kown that the Fe-Al mixture particles during ball-milling process experience strong plastic deformation due to violent impact collision and mutual rolling, the density of solid defects dramatically increase as a result that the particles and substructures are continuously refined. The high-density defects can provide a particular channel for solid-state diffusion between Fe and Al atoms, while the refined substructure can greatly shorten the atomic diffusion distance. In addition, the density of interfacial area is increased with increasing ball-milling time [26]. The large interfacial area between particles means high surface energy and energy stored. Furhermore, the increased temperature of powder mixture due to mutual impact among grinding balls during ball-milling is also benefical to the diffusion of Fe and Al atoms. Therefore, the solid state diffusion between Fe and Al atoms can be easily activated as a result that a higher solid solution degree is obtained for the Fe-Al powder mixtue. The intermetallic compounds during sintering process are easlily formed at a lower temperature for the ball-milled sample rather than no or inadequate ball-milled one. Therefore, the P1 peak shifting to lower temperature is associated with the increased solid solution degree.
For further understanding the formation mechanism of the IF peaks, the XRD patterns are exhibited in figure 7 for the ball-milled 1 h sample. The XRD samples are firstly heated to 480 °C, 565 °C, 635 °C and 675 °C corresponding to the end temperature of P1, P2, P3 and P4 IF peaks respectively and then cooled to room temperature. It can be found that the FeAl3 and Fe2Al5 phase can be identified in figure 7(b), but main compositions remain elementary substance Fe and Al after 480 °C sintering. Increasing temperature up to 565 °C, many diffraction peaks of Fe2Al5 phase can be noticed after appearance of P2 peak, indicated the formation of Fe2Al5 phase. When the temperature reaches to 635 °C, FeAl2 phase can be noted. Finally, almost all intermetallic compounds and residual Fe and Al are transisted into ordered FeAl phase after 675 °C sintering. The results are well matched with the IF features, disclosed that the appearance of four IF peaks are probably originated from the phase formation according to following phase transformation sequence: Fe + Al → FeAl3 → Fe2Al5 → FeAl2 → FeAl during sintering process for the binary Fe-Al elemental mixtue.
Figure 7. XRD patterns of the Fe-Al powder compact sintered at different end temperature of four IF peaks.
Download figure:
Standard image High-resolution image4. Conclusions
Combined with XRD, DSC patterns analysis and SEM observation, the IF spectrum was firstly applied to investigate the phase transformation behaviors of Fe-Al powder mixture during sintering process. Special attentions are concentrated on the influence of mechanical ball-milling on phase transition. Four typical IF peaks were found during sintering process for the ball-milled Fe-Al powder compact, termed P1, P2, P3 and P4 peak respectively. As a comparison, there are only two IF peaks appeared in no ball-milled one. It was rationalized that the appearance of the four IF peaks are respectively associated with the formation of intermetallic compounds FeAl3, Fe2Al5, FeAl2 and FeAl alloys arose from solid diffusion reaction, which is well matched with the phase formation sequence in light of binary phase diagram. Mechanical ball-milling exhibits an important influence on the phase transformation process of Fe-Al powder mixture. The longer milling time is favorable to the phase formation and results in corresponding IF peak shifting towards lower temperature. The reasons can be considered to originate from the enhancement of solid solution degree due to more refined powder particle and substructure as well as increased solid defect density and interfacial area. The higher solid solution degree means that the solid diffusion reaction can be activated at lower temperature. The absence of two IF peaks in no ball-milled sample is ascribed to the lower solid solution degree as the phase transformation takes place only at higher temperature.
Acknowledgments
The present work was supported by the National Natural Science Foundation of China (No. 51661032, 52061038 and 51301150) and Special Program of Science and Technology New Star of Shaanxi Province (No. 2013KJXX-11).
Conflict of interest
The authors declare no conflicts of interest.