Abstract
In order to realize the green and low-cost industrial production of acetylated lignin, this work proposes the heterogeneous reaction to acetylated lignin (ACAL) without catalysts and solvents at high temperature. The influence of reaction temperature and reaction time were investigated by IR, UV–vis, thermogravimetric analysis and water contact angle. Results showed that the optimum technological conditions were about 150 °C and 6 h. The degree of acetylation and the amount of residual phenolic hydroxyl groups of ACAL was 2.49 and 34.2%, respectively. Compared with conventional acetylated lignin, the ACAL had similar hydrophobicity with a water contact angle of 62.0°. The DTG peak of ACAL at about 200 °C reduced to 0.07 than the traditional acetylated lignin. The tensile strength and elongation of poly-lactic acid with 5 wt% ACAL increased to 64.03 MPa and 10.80%, respectively. ACAL revealed a great potential for mass production and applications owing to the eco-friendly and cost-effective modified method.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Biomaterials research has become a very important growing trend now with the decrease of fossil resources [1]. In current biomass materials, lignin is the most abundant and renewable aromatic polymer. However, lignin, as a by-product of the pulp industry, has usually been burnt as liquid waste and not utilized effectively. Therefore, modifying lignin to prepare lignin-based composite polymers is a valid method to realize lignin's use for high value [2–4]. Among the present derivatives of lignin, acetylated lignin, as an important lignin-derivatives with promising application prospects, is widely used to enhance the performance of plastics [5, 6].
However, because of the low reaction activity of hydroxyl groups, the acetylation reaction of lignin has usually used catalysts, solvents, and excess reaction reagents at room temperature, or used acetyl chloride and acetyl bromide to increase its reaction rate [7]. Obviously, these traditional technologies were not suitable for industrial application of lignin because of the high toxicity of the acetyl donor, difficult recovery of the catalysts, and solvents, and use of acetylation agents in excessive quantities. A precipitation agent was also necessary for the separation of the product, because the produced lignin ester dissolved in the excessive acetic anhydride (Ac2O). The complex purification steps raise the production costs, and cause environmental pollution. At present, some novel technology has been proposed to reduce the use of catalysts and solvents by using liquid-phase synthesis [8], mechanical-activation [9], microwave-assisted [10], supercritical CO2 [11] or ionic liquid [12], while these novel methods were costly and did not eliminate the mentioned problems above.
In this paper, acetylated lignin (ACAL) was fabricated at high temperature without catalysts, solvents, excessive Ac2O and complex purification steps. The reaction processes and properties of ACAL were studied and compared with conventional acetylated lignin, by IR spectra, UV–vis spectra, water contact angle and thermogravimetric analysis. Poly-lactic acid (PLA) was chosen to blends with ACAL to evaluate the potential of the ACAL applied in nonpolar polyester. This study aims to propose an eco-friendly and cost-efficient method for mass production and industrial utilization of lignin.
2. Experimental section
2.1. Materials
Alkali lignosulfonate (AL), product number L0082, pH 8.0--10.0, was purchased from TCI Shanghai Co., Ltd (Shanghai, China). AL dried in a vacuum oven at 60 °C overnight. Acetic anhydride (analytical regent grade) was purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China) and was used without further purification. PLA 290, was injection grade and purchased from Zhejiang Hisun Biomaterials Co., Ltd.
2.2. Preparations
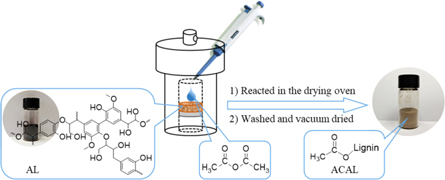
A typical lignin acetylated procedure is shown in figure 1. Dry AL (1 g) was first added to a crucible filter, followed by dropping 1.2 times (w: w) of Ac2O. The crucible filter was then sealed in a hydrothermal synthesis reactor and reacted in the oven for some hours at high temperature. The product was ground and washed with distilled water three times. Finally, the product was milled to pass through a 200-mesh sieve and dried in a vacuum chamber at 60 °C for 1 d. The experiments under different conditions were shown in table 1.
Figure 1. Scheme of AL heterogeneous acetylation reaction.
Download figure:
Standard image High-resolution imageTable 1. Effect of reaction temperature and time on degree of acetylation and amounts of residual phenolic hydroxyl groups.
| Sample | Temperature (°C) | Reaction time (h) | Acetylating agents | Degree of acetylation | Residual phenolic-OH (%) |
|---|---|---|---|---|---|
| ACAL-1 | 130 | 1 | 1.40 | 61.0 | |
| ACAL-2 | 130 | 24 | 2.64 | 30.5 | |
| ACAL-3 | 150 | 1 | 1.76 | 58.9 | |
| ACAL-4 | 150 | 6 | 2.49 | 34.2 | |
| ACAL-5 | 150 | 24 | 2.41 | 30.7 | |
| ACAL-6 | 170 | 1 | Ac2O | 1.99 | 63.0 |
| ACAL-7 | 170 | 6 | 2.41 | 36.4 | |
| ACAL-8 | 170 | 24 | 2.37 | 33.0 | |
| ACAL-9 | 190 | 1 | 2.15 | 58.3 | |
| ACAL-10 | 190 | 3 | 2.24 | 40.1 | |
| ACAL-11 | 190 | 9 | 2.15 | 29.6 |
Two control samples were prepared for comparison according to the conventional acetylation methods from literature. CAL-1: 0.1 g AL was added to a flask containing 10 ml acetylation reagent (acetyl bromide to glacial acetic acid, v/v, 8:92) stirred at 50 °C for 2 h. CAL-2: 0.1 g AL, 1 ml pyridine and 1 ml acetic anhydride were added to flask and stirred for 72 h at room temperature.
A HAAKE Mini-CTW (Thermo Scientific Corp.) cone twin-screw extruder was used to prepare the composites. PLA was blended with ACAL-4 at 160 °C, 50 rpm and recirculated for 5 min The produced composites were labeled PLA/ACAL-4. The control samples of PLA/AL, PLA/CAL-1 and PLA/CAL-2 were also prepared in a similar procedure.
For mechanically testing the composites, dumbbell specimens were prepared in accordance with ASTM D638-14 IV type. The specimens were obtained by injection molding with a HAAKE Mini jet II (Thermo Scientific Corp.), under conditions of a cylinder temperature of 180 °C, mold temperature of 60 °C, injection pressure of 75 MPa for 12 s, and post pressure of 45 MPa for an additional 8 s.
2.3. Characterization
Fourier-transform infrared (FT-IR) analyses were performed on a Nicolet-7600 spectrometer (Thermo Scientific Corp.). The samples were combined with potassium bromide and the mixture was pressed into a disk for testing. The spectra was recorded with 32 scans between 400 and 4000 cm−1 at room temperature. The ratio of the carbonyl absorbance bands and characteristic band of the aromatic skeletal vibrations can be used to estimate the content of acetyl groups, according to the reference [13], hence, the degrees of acetylation (DA) were estimated by the equation (1):
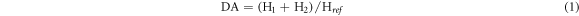
Where H1, H2 and H ref represent the intensity of the absorbed bands at approximately 1743, 1765, and 1508 cm−1, respectively.
Ultraviolet–visible (UV–vis) spectroscopy analysis was conducted on Lambda 25 spectrometer (PerkinElmer Corp.) and was recorded with 120 nm min−1 scans between the wavelengths of 200–600 nm. According to the references [14], about 5–15 mg samples were dissolved in 10 ml of water and a 1, 4-dioxane (v: v = 1: 9) mixed solutions. Then the 1 ml solution was diluted to 25 ml neutral (pH = 6) and alkaline (pH = 14) buffer solution, respectively. The neutral solution without sample was used to calibration baseline. Ionization of phenolic OH in lignin is used for qualitative and quantitative analyses of lignin. Lignin structures are grouped into six types in the reference and each type of phenolic group absorptivity at different wavelengths and its quantity can be evaluated by the following equation (2)–(5):
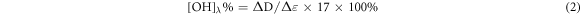
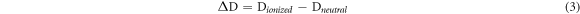
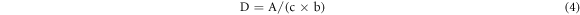
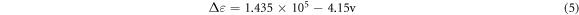
Where [OH]λ % is phenolic hydroxyl groups of each types, ΔD is the specific difference absorptivity, D is the specific absorptivity (Lg−1cm−1), A is absorbance, c is the concentration (g/L), b is length of light path way (cm), and Δε is the difference in the molar absorptivity and obtained according to the empirical equation, where v is the wave number (cm−1).
Water contact angle was conducted on a SDC-350 Contact Angle Meter (SINDIN Corp.) and measured with sessile drop. About 0.06 g powder samples were pressed in to disk under 15 MPa for testing. The values quoted are the average of three measurements carried out with deionized water.
Thermogravimetric analysis was performed on STA-2500 (Netzsch Corp.) under a N2 atmosphere from 30 °C to 600 °C, at a heating rate of 10 K min−1, and a flow rate of 30 ml min−1.
The PLA blends were mechanically tested using an electronic universal testing machine (UTM6503, SUNS Co., Ltd) equipped with a load cell of 5 kN, at a speed of 10 mm min−1. The values quoted are the average of three measurements.
3. Results and discussion
3.1. Effect of reaction time and reaction temperature
The effect of reaction time and reaction temperature of degree of acetylation and amount of non-acetylated hydroxyl groups were shown in table 1, respectively. The acetylation reaction at 130 °C was slow and took 24 h for maximum DA value to reach approximately 2.61. When the reaction temperature was raised to 150 °C and 170 °C, the maximum DA values reached 2.51 and 2.44 after 6 h, respectively. Increasing reaction time, their DA values were nearly invariable. As theory, the amount of residual phenolic hydroxyl decreased with the DA value increasing. However, when the reaction time went from 6 to 24 h, the amount of phenolic hydroxyl of ACAL-5 was lower than ACAL-4. The similar phenomenon happened at reaction temperature of 190 °C, the reaction rate was much faster, while the maximum DA value was only 2.29. When raised reaction time, the DA value decreased obviously. These may be related to the free-radical reactions as condensation and deconstruction of ACAL affected by the acid condition and high temperature [15, 16]. Hence, considering the treating efficiency and effect, 150 °C was selected as the optimum reaction temperature for ACAL.
3.2. Effect of different acetylation methods
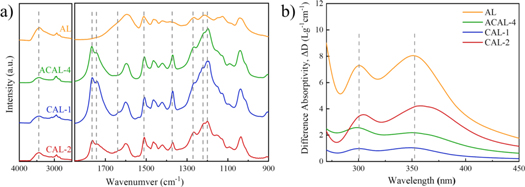
The ACAL and control samples were compared by the FT-IR spectra shown in figure 2(a). The crude lignin showed a typical lignosulfonates IR spectra and was recognized according to the literature [17]. 1265 cm−1 and 1220 cm−1 band was attributed to C–H plane vibrations and C–O–C, respectively, and meant the raw material is G-type lignin. All acetylated samples showed intense bimodal signals at approximately 1743 and 1765 cm−1 caused by carbonyl group stretching, which are assigned to aliphatic acetyl and phenolic acetyl, respectively. The enhancing of bands at 1371 cm−1 and 1200 cm−1 corresponded to aliphatic C–H stretch in CH3 and C–O vibration of acetyls, respectively, also caused by the substitutions of acetyl. The band at 3440 cm−1 attributed to the O–H stretching decreased upon acetylation, but still observed in spectra, indicated that hydrogen bonding and steric hindrance prevent acetylation of hydroxyls [18]. The three characteristic peaks belonging to the aromatic skeletal vibration were at 1600cm−1, 1508 cm−1 and 1420 cm−1, respectively. The aromatic ring structure of lignin is considered to be stable during acetylation reactions. In general, different acetylation methods showed a similar IR spectra of characteristic band. However, the traditional acetylated lignin samples (CAL-1 and CAL-2) had a band of 1635cm−1 which was attributed to C=O stretch from conjugated p-substituent carbonyl, and the band was hardly visible on ACAL.
Figure 2. (a) FTIR spectra and (b) ionization difference spectrum of raw lignin (AL), heterogeneous reaction at 150 °C for 6 h (ACAL-4) and conventional acetylated lignin (CAL-1 and CAL-2).
Download figure:
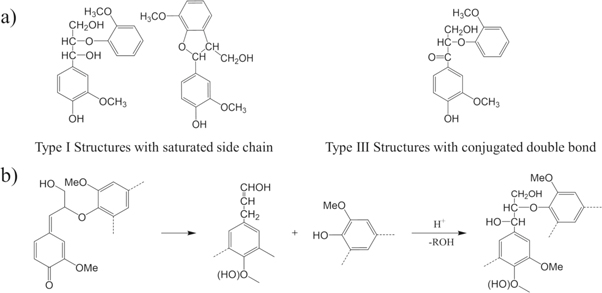
Standard image High-resolution imageThe difference between ACAL and CAL was also studied by the ionization difference spectra. According to figure 2(b). The absorption peak at 300 nm and 353 nm corresponded to type 1 and type 3 phenolic structures (figure 3(a)). The absorption at 353 nm of AL, CAL-1 and CAL-2 were stronger than that at 300 nm. While that of ACAL-4 exhibited a higher peak at 300 nm than 353 nm. Hence in, figure 3(b) showed possible mechanism of structure changes of ACAL, according to the literature [15, 19].
Figure 3. (a) Types 1 and type 3 phenolic structures according to reference [20], reprinted by permission from Springer Nature; (b) proposed mechanism of structure changes of ACAL.
Download figure:
Standard image High-resolution image3.3. Water contact angle
The water contact angle revealed the hydrophobic properties of acetylated lignin. The improvement of hydrophobicity contributes to the compatibility of acetylated lignin with nonpolar polymers. The value of contact angle of AL was around 32.0 ± 2.9°, which could relate to the high content of OH groups since OH groups were hydrophilic. With reaction time extending, the value of contact angle was 53.8 ± 2.4°, 62.0 ± 0.5°and 58.3 ± 1.8°, respectively, and more related to the value of degree of acetylation than the residual hydroxyl groups. As results shown in figure 4(b), the contact angle between different acetylation methods also conformed to the laws, the values of ACAL-4, CAL-1 and CAL-2 were 62.0 ± 0.6°, 69.6 ± 1.8° and 60.2 ± 4.3°, respectively. The ACAL had similar hydrophobic property with traditional acetylation lignin, while the preparation of ACAL was green and low-cost. In combination with results that had been discussed above, the optimum reaction time and reaction temperature was about 6 h and 150 °C.
Figure 4. Influence of (a) reaction time (b) different reaction methods on water contact angle.
Download figure:
Standard image High-resolution image3.4. Thermal properties
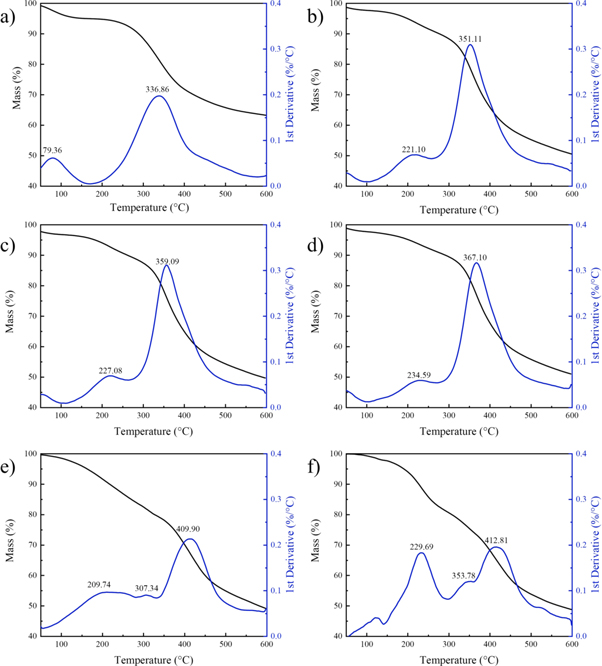
The thermogravimetric analysis (TGA) and the derivative thermogravimetric analysis (DTG) results for AL, ACAL and control samples are shown in figure 5. Compared to the TGA curves of AL and acetylated lignin, pyrolysis residue contents of acetylated sample were reduced from that of AL, indicating that the introduction of acetyl groups inhibited the coke formation. Compared to the DTG curves of AL and ACAL, before 150 °C, the reduction of sample mass was due to the removal of free water [21]. The substitution of hydroxyl by acetyl reduced the hydrophilicity of lignin and had proved by the water contact angle above. A new degradation stage appeared around 150 to 250 °C upon acetylation, the new stage was recognized as the degradation of the side chain of lignin structures [22]. The introduction of acetyl groups reduced hydrogen bonds interaction of lignin, thus promoting the pyrolysis efficiency of ACAL [23, 24]. The third stage was the decomposition stage of the majority. At this stage, the DTG peak of ACAL shifted from 351.11 to 367.10 °C, indicating that the thermal stability of ACAL increasing with reaction time extending. Influence of different acetylation methods shown in figure c, e and f, at the peak about 200 °C–250 °C, the instantaneous decomposition rate peak of ACAL-4, CAL-1 and CAL-2 were 0.07, 0.1 and 0.182, respectively. Hence, the thermal stability of ACAL-4 increased by 43%–160% than conventional acetylated lignin. The small peaks at about 300 °C–350 °C only appeared in CAL-1 and CAL-2 derivative curves but not appeared in ACAL, which was caused by the degradation of the acetylated units of lignin [25], which implied that the conventional acetylation methods weakened the intermolecular interactions of lignin. Herein, the better thermal stability of ACAL could be attributed to the self-condensation reactions on the lignin backbone, through the cross-linking and strong intermolecular interactions at high reaction temperature. The better thermal stability of ACAL increases the window of process ability and ranges of applications.
Figure 5. TGA and DTG of (a) AL; (b) ACAL-3; (c) ACAL-4; (d) ACAL-5; (e) CAL-1; (f) CAL-2.
Download figure:
Standard image High-resolution image3.5. Mechanical properties of ACAL-PLA composites
PLA with 5% ACAL or CAL blends were mechanically tested and shown in table 2. The mechanical properties of PLA/ACAL and PLA/CAL were all better than PLA/AL, which indicated that acetylation improved compatibility with PLA. The tensile strength of the PLA/CAL-1 and PLA/CAL-2 composites is lower than that of PLA, while the PLA/ACAL-4 were almost the same with neat PLA. In addition, the elongations of PLA/ACAL were slightly less than PLA/CAL, in general, raised with DA value increasing. However, CAL-2 with low DA value had similar mechanical properties with CAL-1 in PLA matrix, could result from the difference in structure and morphology caused by two acetylation approaches. Young's modulus of PLA/ACAL-4 showed a little decrease than other samples. As many researchers reported, the lignin esters performed for plasticizers for polymers [26–29]. Compared with CAL-1 and CAL-2, ACAL-4 exhibited a good balance of strength and toughness.
Table 2. Results of tensile tests for ACAL-PLA blends (5% Modified Lignin Content).
| Sample | Tensile strength (MPa) | Elongation at break (%) | Young's Modulus (MPa) |
|---|---|---|---|
| PLA | 63.63 ± 1.68 | 6.79 ± 0.18 | 893.35 ± 104.09 |
| PLA/AL | 52.88 ± 7.51 | 7.87 ± 2.08 | 738.48 ± 33.97 |
| PLA/ACAL-4 | 64.03 ± 3.20 | 10.80 ± 1.13 | 770.51 ± 214.66 |
| PLA/CAL-1 | 58.81 ± 1.74 | 12.08 ± 0.54 | 1061.36 ± 31.81 |
| PLA/CAL-2 | 58.09 ± 1.59 | 12.46 ± 1.27 | 858.34 ± 196.66 |
4. Conclusions
In this study, ACAL was prepared by a high temperature heterogeneous esterification reaction. The novel process made ACAL remain in solid state after reaction for facile purification. Results showed that, the optimum technological conditions were about 150 °C and 6 h. The degree of acetylation and the amount of residual phenolic hydroxyl groups of ACAL were 2.49 and 34.2%, respectively. Compared with conventional acetylated lignin, the ACAL had similar hydrophobicity with a water contact angle of 62.0°. The DTG peak of ACAL at about 200 °C reduced to 0.07 than the traditional acetylated lignin. By mechanical testing, the tensile strength and elongation of PLA with 5 wt% ACAL increased to 64.03 MPa and 10.80%, respectively. In general, the solvents-free, catalyst-free, reagent saving and separation easily method for ACAL is green and cost-effective, showing a good prospect for industrial application.
Acknowledgments
The authors are grateful for the project funded by National Key Research and Development Program of China (2020YFE0100100), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Co-Innovation Center for Efficient Processing and Utilization of Forest Products, College of Chemical Engineering, Nanjing Forestry University.
Conflicts of interest
The authors declare no conflict of interest.