Abstract
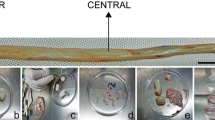
The current study investigated the change in umbilical cord tissue and the number of markers of Wharton’s jelly mesenchymal stem cells (WJ-MSC) in pregnant women with gestational diabetes (GDM), with chronic diabetes who developed nephropathy as vascular complication (VC-PGDM), and healthy pregnant women as the control. The umbilical cords (UC) were investigated by the histomorphological method and the number of WJ-MSC were detected by flow-cytometry using the CD90, CD44, CD105, and CD73 markers in Wharton’s jelly (WJ) isolated from fresh umbilical cords. The number of positive cells for CD 90, CD44, CD105, and CD73 were found to be elevated in the GDM group, whereas it was significantly diminished in the VC-PGDM group (p = 0.001, p = 0.001, p = 0.001, and p = 0.001). The only histopathological sign in the GDM group were an increased number of pores in the Wharton jelly. Artery wall thickness/cord diamater ratio was increased, which indicates an increase of the artery wall thickness in the VC- PGDM group (p = 0.039 and p = 0.048). The increase in umbilical cord diameter and number of Wharton jelly mesenchymal stem cells in babies of gestational diabetic mothers was considered as an effect of macrosomia seen in babies of mothers with gestational diabetes. Vasculopathy, a long-term complication of diabetes, is known to affect all tissues by causing marked lower perfusion and hypoxia, as well as a decrease in the MSC number in our study.





Similar content being viewed by others
References
White P (1978) Classification of obstetric diabetes. Am J Obstet Gynecol 130(2):228–230
Mayhew T (2002) Enhanced fetoplacental angiogenesis in pre-gestational diabetes mellitus: the extra growth is exclusively longitudinal and not accompanied by microvascular remodelling. Diabetologia 45(10):1434–1439
Verma R, Mishra S, Kaul JM (2010) Cellular changes in the placenta in pregnancies complicated with diabetes. Int J Morphol 28(1):259–264
Haeri S, Khoury J, Kovilam O, Miodovnik M (2008) The association of intrauterine growth abnormalities in women with type 1 diabetes mellitus complicated by vasculopathy. Am J Obstetr Gynecol 199(3):278. e1–278. e5.
Dunne FP (1999) Pregestational diabetes mellitus and pregnancy. Trends Endocrinol Metab 10(5):179–182
Arvas A (1993) Diyabetik Anne Bebeği. Perinatoloji Dergisi 1:122–127. https://www.perinataljournal.com/Files/Archive/tr-TR/Articles/PD-19930012013tr.pdf
Bourbon JR, Farrell PM (1985) Fetal lung development in the diabetic pregnancy. Pediatr Res 19(3):253–267
Piazze JJ, Anceschi MM, Maranghi L, Brancato V, Marchiani E, Cosmi E (1999) Fetal lung maturity in pregnancies complicated by insulin-dependent and gestational diabetes: a matched cohort study. Eur J ObstetrGynecolReprod Biol 83(2):145–150
Bongso A, Fong C-Y (2013) The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev Rep 9(2):226–240
Wajid N, Nseem R, Anwar SS, Awan SJ, Ali M, Javed S (2015) The effect of gestational diabetes on proliferation capacity and viability of human umbilical cord-derived stromal cells. Cell Tissue Bank 16(3):389–397
Kim J, Piao Y, Pak Y et al (2015) Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev 24(5):575–586
Alam R, Momen A, Sultana AA, Hassan N (2014) Gross and Histomorphologic study of the umbilical cord in pre-gestational Diabetes mellitus and gestational Diabetes mellitus. Bangladesh J Anat 12(1):25–29
Ishibashi H, Suzuki T, Suzuki S et al (2003) Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab 88(5):2309–2317
Schwartz R, Teramo KA (2000) Effects of diabetic pregnancy on the fetus and newborn. Semin Perinatol 24(2):120–135
Dilbaz S, Gelişen O, Dilbaz B et al (2004) Diabetik Gebeliklerde Diabet Tipinin Doğum Şekline, Doğum Ağirliğina Ve Apgar Skoruna Etkisi. Kadın Doğum Dergisi 2(4):275–277. https://www.journalagent.com/goj/pdfs/GOJ_2_4_250_323.pdf#page=31
Arieh Riskin MAG-P (2020) Infants of women with diabetes. 2020 [cited 2020 10.04.202]; https://www.uptodate.com/contents/infants-of-women-with-diabetes
Ekici F, Yıldırım A, Sevim Ü et al (2010) Diyabetik anne bebeklerinin kardiyovasküler sistem hastalıkları ve izlemi. Gazi Med J 21(2):
Akarsu S, Çıtak Kurt N, Kurt A, Yımaz E, Aygün D (2008) Diyabetik anne bebeğinde klinik ve laboratuar bulguları. Fırat Tıp Dergisi 13(3):199–204. https://www.firattipdergisi.com/pdf/pdf_FTD_500.pdf
Evers IM, de Valk HW, Visser GH (2004) Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 328(7445):915
Jensen DM, Damm P, Moelsted-Pedersen L et al (2004) Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 27(12):2819–2823
Saraç E (2015) Annelerinde Gestasyonel Diyabet Tanisi Olan Yenidoğanlarin Değerlendirilmeleri, in Pediatri. Dissertation. Abant İzzet Baysal Üniversitesi
Daskalakis G, Marinopoulos S, Krielesi V et al (2008) Placental pathology in women with gestational diabetes. Acta Obstetr Gynecol Scand 87(4):403–407
Taricco E, Radaelli T, Santis MS, Cetin I (2003) Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta 24(4):343–347
Binbir B, Yeniel A, Ergenoglu A et al (2012) The role of umbilical cord thickness and HbA1c levels for the prediction of fetal macrosomia in patients with gestational diabetes mellitus. Arch Gynecol Obstet 285(3):635–639
Kaya S (2015) Gestasyonel diyabet, preeklampsi, hellp sendromu ve normal gebelerde göbek kordonu histopatolojik değişiklikleri. Dissertation
Al-Kazzaz MM, Al-Hubaity A-JY (2000) Ultrastructural vascular changes of the human umbilical cord of new-born babies from diabetic mothers. Iraqi J Med Sci, p 37. https://www.colmed-alnahrain.edu.iq/iraqijms/Iraqi%20JMS%20-%20Volume%202%20(3)%202003/HTML/files/assets/downloads/publication.pdf#page=44
Weissman A, Jakobi P (1997) Sonographic measurements of the umbilical cord in pregnancies complicated by gestational diabetes. J Ultrasound Med 16(10):691–694
Blanco MV, Vega RH, Guerri-Guttenberg R et al (2011) Histopathology and histomorphometry of umbilical cord blood vessels. Findings in normal and high risk pregnancies. Artery Res 5(2):50–57
Pierdomenico L, Lanuti P, Lachmann R et al (2011) Diabetes mellitus during pregnancy interferes with the biological characteristics of Wharton’s Jelly mesenchymal stem cells. Open Tissue Eng Regener Med J 4(1):103
Montanucci P, Teresa P, Ilaria P et al (2018) Functional profiles of human umbilical cord-derived adult mesenchymal stem cells in obese/diabetic versus healthy women. Curr Diabetes Rev14(1):24–35. https://www.ingentaconnect.com/content/ben/cdr/2018/00000014/00000001/art00005
Allen DA, Yaqoob MM, Harwood SM (2005) Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem 16(12):705–713
Afroze SH, Kalagiri RR, Reyes M et al (2016) Apoptotic and stress signaling markers are augmented in preeclamptic placenta and umbilical cord. BBA Clin 6:25–30
Belkacemi L, Kjos S, Nelson DM, Desai M, Ross MG (2013) Reduced apoptosis in term placentas from gestational diabetic pregnancies. J Dev Origins Health Dis 4(3):256–265
Fijany A, Sayadi RL, Khoshab N et al (2019) Mesenchymal stem cell dysfunction in diabetes. Mol Biol Rep 46(1):1459–1475
Kong C-M, Subramanian A, Biswas A et al (2019) Changes in stemness properties, differentiation potential, oxidative stress, senescence and mitochondrial function in Wharton’s Jelly stem cells of umbilical cords of mothers with gestational diabetes mellitus. Stem Cell Reviews and Reports 15(3):415–426
Athapathu H, Jayawardana M, Senanayaka L (2003) A study of the incidence of apoptosis in the human placental cells in the last weeks of pregnancy. J Obstet Gynaecol 23(5):515–517
Funding
This study was funded by Gaziantep University Scientific Research Projects with project number TF.UT.18.04.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Çiğdem Karaca, Nuray Bostancıeri, Ali Ovayolu and Demet Taşdemir Karahan. The first draft of the manuscript was written by Çiğdem Karaca and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Yes.
Consent for publication
All authors approved the publication.
Ethical approval
Permission was obtained with the decision of Gaziantep University Faculty of Medicine Medical Ethics Committee dated 24.07.2017 and numbered 2017/268. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2020_5965_MOESM3_ESM.jpg
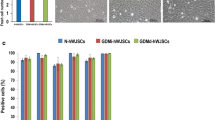
Supplementary Figure 3 Graph comparing CD90, CD105, CD73 and CD44+ cell numbers. All values are represented as mean ± SD.). *Denote significant differences at the level of p<0.005 between the groups (JPG 47 kb)
Rights and permissions
About this article
Cite this article
Karaca, C., Bostancıeri, N., Ovayolu, A. et al. The effect of vascular complications of diabetes mellitus on human umbilical cord tissue and the number of Wharton Jelly’s mesenchymal stem cells. Mol Biol Rep 47, 9313–9323 (2020). https://doi.org/10.1007/s11033-020-05965-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05965-8