Abstract
Microwave-assisted one-pot quick synthesis to 1-monosubstituted 1,2,3-triazoles was achieved with good to excellent yields using the widely available arylboronic acids, sodium azide and 3-butyn-2-ols within 15 min. This method features high efficient and facile as organic azides, acetylene gas and harsh conditions were avoided.
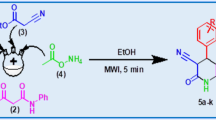
Graphic abstract
Microwave-assisted one-pot quick synthesis to 1-monosubstituted 1,2,3-triazoles was achieved with good to excellent yields using the widely available arylboronic acids, sodium azide and 3-butyn-2-ols within 15 min.





Similar content being viewed by others
References
(a) Lu G, Li X, Mohamed K, Wang D and Meng F 2019 Design, synthesis and biological evaluation of novel uracil derivatives bearing 1, 2, 3-triazole moiety as thymidylate synthase (TS) inhibitors and as potential antitumor drugs Eur. J. Med. Chem. 171 282; (b) Thirumurugan P, Matosiuk D and Jozwiak K 2013 Click chemistry for drug development and diverse chemical-biology applications Chem. Rev. 113 4905
(a) Liu Y, He C, Tang Y, Imler G, Parrish D and Shreeve J 2019 Tetrazolyl and dinitromethyl groups with 1,2,3-triazole lead to polyazole energetic materials Dalton Trans. 48 3237; (b) Chu C and Liu R 2011 Application of click chemistry on preparation of separation materials for liquid chromatography Chem. Soc. Rev. 40 2177
(a) Bozorov K, Zhao J and Aisa H 2019 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview Bioorg. Med. Chem. 27 3511; (b) Johansson J, Beke-Somfai T, Stalsmeden A and Kann N 2016 Ruthenium-catalyzed azide alkyne cycloaddition reaction: scope, mechanism, and applications Chem. Rev. 116 14726; (c) Sheng C and Zhang W 2011 New lead structures in antifungal drug discovery Curr. Med. Chem. 18 733; (d) Jiang Y and Kuang C 2013 Recent advances in the synthesis of 1-monosubstituted 1,2,3-triazoles Mini-Rev. Med. Chem. 13 713
(a) Chen K, Barve I and Sun C 2020 Catalyst-controlled regioselective synthesis of benzotriazolodiazepin-7-ones and benzotriazolodiazocin-8-ones Org. Lett. 22 428; (b) Virant M and Kosmrlj J 2019 Arylation of click triazoles with diaryliodonium salts J. Org. Chem. 84 14030; (c) Ren Y, Liu Y, Gao S, Dong X, Xiao T and Jiang Y 2020 Palladium-catalyzed selective ortho C-H alkoxylation at 4-aryl of 1,4-disubstituted 1,2,3-triazoles Tetrahedron 76 130985; (d) Zhao F, Liu Y,Yang S, Xie K and Jiang Y 2017 Pd-catalyzed selective N(3)-ortho C-H arylation of 1,4-disubstituted 1,2,3-triazoles Org. Chem. Front. 4 1112; (e) Gonzalez-Mojica N, Almazan-Sanchez L, Garcia-Torres J, et al. 2019 Oxidation of 1,4-disubstituted-1,2,3-triazoles with H2O2-CF3CO2H: efficient synthesis of 1,2,3-triazole 3-oxides Synth. Commun. 49 679; (f) Ma X, Li H, Xin H, Du W, Anderson E, Dong X and Jiang Y 2020 Copper-catalyzed intramolecular C-H alkoxylation of diaryltriazoles: Synthesis of tricyclic triazole benzoxazines Org. Lett. 22 5320; (g) Wang J, Yang J, Fu X, Qin G, Xiao T and Jiang Y 2019 Synthesis of triazole-fused phenanthridines through Pd-catalyzed intramolecular phenyl C-H activation of 1,5-diaryl-1,2,3-triazoles Synlett 30 1452
(a) Rohrig U, Majjigapu S, Grosdidier A, et al. 2012 Rational design of 4-aryl-1,2,3-triazoles for indoleamine 2,3-dioxygenase 1 inhibition J. Med. Chem. 55 5270; (b) Rohrig U, Reynaud A, Majjigapu S, et al. 2019 Inhibition mechanisms of indoleamine 2,3-dioxygenase 1 (IDO1) J. Med. Chem. 62 8784
(a) Totir M, Padayatti P, Helfand M, et al. 2006 Effect of the inhibitor-resistant M69V substitution on the structures and populations of trans-enamine β-lactamase intermediates Biochemistry 45 11895; (b) Zhang B 2019 Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids Eur. J. Med. Chem. 168 357; (c) Fer M, Le Corre L, Pietrancosta N, et al. 2018 Bacterial transferase MraY, a source of inspiration towards new antibiotics Curr. Med. Chem. 25 6013
(a) Bian J, Zhang L, Han Y, Wang C and Zhang L 2015 Histone deacetylase inhibitors: Potent anti-leukemic agents Curr. Med. Chem. 22 2065; (b) Kalinin D, Jana S, Pfafenrot M, et al. 2020 Structure-based design, synthesis, and biological evaluation of triazole-based smHDAC8 inhibitors ChemMedChem 15 571
(a) El Akri K, Bougrin K, Balzarini J, Faraj A and Benhida R 2007 Efficient synthesis and in vitro cytostatic activity of 4-substituted triazolyl-nucleosides Bioorg. Med. Chem. Lett. 17 6656; (b) Bonandi E, Christodoulou M, Fumagalli G, et al. 2017 The 1,2,3-triazole ring as a bioisostere in medicinal chemistry Drug Discov. Today 22 1572
Huisgen R 1961 centenary lecture. 1,3-Dipolar cycloadditions Proc. Chem. Soc. 357
Rostovtsev V, Green L, Fokin V and Sharpless K 2002 A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes Angew. Chem. Int. Ed. 41 2596
(a) Neto J and Zeni G 2020 A decade of advances in the reaction of nitrogen sources and alkynes for the synthesis of triazoles Coordin. Chem. Rev. 409 213217; (b) Autade S and Akamanchi K 2019 Sulfated tungstate a heterogeneous acid catalyst for synthesis of 4-aryl-NH-1,2,3-triazoles by 1,3-dipolar cycloaddition of nitroolefins with NaN3 Synth. Commun. 49 1947; (c) Jiang Y and Kuang C 2012 Synthesis of 1,2,3-triazole derivatives Prog. Chem. 24 1983
(a) Kwok S, Fotsing J, Fraser R, Rodionov V and Fokin V 2010 Transition-metal-free catalytic synthesis of 1,5-diaryl-1,2,3-triazoles Org. Lett. 12 4217; (b) Cheng X, Liu W and Huang Z 2013 Sodium hydride-mediated synthesis of 1,5-diaryl-1,2,3-triazoles from anti-3-aryl-2,3-dibromopropanoic acids and organic azides Chin. Chem. Lett. 24 764; (c) Maiuolo L, Russo B, Algieri V, et al. 2019 Regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles by 1,3-dipolar cycloaddition: Role of Er(OTf)3, ionic liquid and water Tetrahedron Lett. 60 672
(a) Gonzalez-Calderon D, Santillan-Iniesta I, Gonzalez-Gonzalez C, et al. 2015 A novel and facile synthesis of 1,4,5-trisubstituted 1,2,3-triazoles from benzylic alcohols through a one-pot, three-component system Tetrahedron Lett. 56 514; (b) Guo N, Liu X, Xu H, et al. 2019 A simple route towards the synthesis of 1,4,5-trisubstituted 1,2,3-triazoles from primary amines and 1,3-dicarbonyl compounds under metal-free conditions Org. Biomol. Chem. 17 6148; (c) Chen Z, Yan Q, Liu Z, Xu Y and Zhang Y 2013 Copper-mediated synthesis of 1,2,3-triazoles from N-tosylhydrazones and anilines Angew. Chem. Int. Ed. 52 13324; (d) Chen Z, Liu Z, Cao G, et al. 2017 Recent advances in multicomponent synthesis of 1,4,5-trisubstituted 1,2,3-triazoles Adv. Synth. Catal. 359 202
Wu L, Xie Y, Chen Z, Niu Y and Liang Y 2009 A convenient synthesis of 1-substituted 1,2,3-triazoles via CuI/Et3N catalyzed ‘click chemistry’ from azides and acetylene gas Synlett 1453
(a) Andersen J, Bolvig S and Liang X 2005 Efficient one-pot synthesis of 1-aryl 1,2,3-triazoles from aryl halides and terminal alkynes in the presence of sodium azide Synlett 2941; (b) Chan D, Laughton C, Queener S and Stevens M 2002 Structural studies on bioactive compounds. Part 36: Design, synthesis and biological evaluation of pyrimethamine-based antifolates against pneumocystis carinii Bioorg. Med. Chem. 10, 3001; (c) Fletcher J, Walz S and Keeney M 2008 Monosubstituted 1,2,3-triazoles from two-step one-pot deprotection/click additions of trimethylsilylacetylene Tetrahedron Lett. 49 7030; (d) Jiang Y, Kuang C and Yang Q 2011 Facile and quick synthesis of 1-monosubstituted aryl 1,2,3-triazoles: a copper-free [3+2] cycloaddition Tetrahedron 67 289; (e) Jiang Y, Kuang C and Yang Q 2009 The use of calcium carbide in the synthesis of 1-monosubstituted aryl 1,2,3-triazole via click chemistry Synlett 3163; (f) Giel M, Smedley C, Mackie E, Guo T, et al. 2020 Metal-free synthesis of functional 1-substituted-1,2,3-triazoles from ethenesulfonyl fluoride and organic azides Angew. Chem. Int. Ed. 59 1181; (g) Shang J, Fu H, Li Y, Yang T, Gao C and Li Y 2019 Copper-catalyzed decarboxylation/cycloaddition cascade of alkynyl carboxylic acids with azide Tetrahedron 75 253; (h) Han C, Dong S, Zhang W and Chen Z 2018 One-Pot Synthesis of 1-monosubstituted 1,2,3-triazoles from propargyl alcohol Synlett 29 673; (i) Liu Y, Han C, Ma X, Yang J, Feng X and Jiang Y 2018 One-pot synthesis of 1-monosubstituted-1,2,3-triazoles from 2-methyl-3-butyn-2-ol Tetrahedron Lett. 59 650; (j) Wu L, Yan B, Yang G and Chen Y 2013 Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water Heterocycl. Commun. 19 397; (k) Haebich D, Barth W and Roesner M 1989 Synthesis of 3’-(1,2,3-triazol-1-yl)-3’-deoxythymidines Heterocycles 29 2083; (l) Kadaba P 1992 Triazolines. 25. 1,3-Cycloaddition of aryl azides to enamides and the synthesis of 1-aryl-5-amido-1,2,3-triazolines J. Org. Chem. 57 3075; (m) Sasaki T, Eguchi S, Yamaguchi M and Esaki T 1981 Synthesis of adamantane derivatives. 52. 1,3-Dipolar cycloaddition reactions of 1-azidoadamantane. Reactivity, regioselectivity, and carbon-13 nuclear magnetic resonance spectra of 1-(1-adamantyl)-Δ2-1,2,3-triazolines and -1H-1,2,3-triazoles J. Org. Chem. 46 1800; (n) Huang Z, Wang R, Sheng S, Zhou R and Cai M 2013 Preparation of polystyrene-supported vinyl sulfone and its application in the solid-phase organic synthesis of 1-monosubstituted 1,2,3-triazoles React. Funct. Polym. 73 224; (o) Naud J, Lemke C, Goudreau N, Beaulieu E and White P 2008 Potent triazolyl-proline-based inhibitors of HCV NS3 protease Bioorg. Med. Chem. Lett. 18 3400; (p) Yang Q, Jiang Y and Kuang C 2012 Facile one-pot synthesis of monosubstituted 1-aryl-1H-1,2,3-triazoles from arylboronic acids and prop-2-ynoic Acid (=propiolic acid) or calcium acetylide (=calcium carbide) as acetylene source Helv. Chim. Acta 95 448; (q) Xu M, Kuang C, Wang Z, Yang Q and Jiang Y 2011 A novel approach to 1-monosubstituted 1,2,3-triazoles by a click cycloaddition/decarboxylation process Synthesis 223; (r) Kolarovic A, Schnurch M and Mihovilovic M 2011 Tandem catalysis: From alkynoic acids and aryl iodides to 1,2,3-triazoles in one pot J. Org. Chem. 76 2613
(a) Yang H, Li Y, Jiang M, Wang J and Fu H 2011 General copper-catalyzed transformations of functional groups from arylboronic acids in water Chem. Eur. J. 17 5652; (b) Tao C, Cui X, Li J, Liu A, Liu L and Guo Q 2007 Copper-catalyzed synthesis of aryl azides and 1-aryl-1,2,3-triazoles from boronic acids Tetrahedron Lett. 48 3525
Acknowledgements
We are grateful for the financial support provided by the Natural Science Foundation of Yunnan Education Department (No. 2020J0768).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DU, Z., LI, F., LI, L. et al. Microwave-assisted one-pot quick synthesis of 1-monosubstituted 1,2,3-triazoles from arylboronic acids, sodium azide and 3-butyn-2-ols. J Chem Sci 132, 154 (2020). https://doi.org/10.1007/s12039-020-01856-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01856-4