Abstract
Hydrogeochemical investigations have been carried out in a semi-arid region of Aland taluk of Karnataka State, India. The analysis has been done to examine the quality of groundwater for drinking, domestic and irrigational purposes. In this concern, thirty-two groundwater samples were collected in pre-monsoon (April 2016) and post-monsoon season (November 2016), from the different location within the study area. These samples have been further analysed for different ions such as CO32−, HCO3−, NO3−, Ca2+, Mg2+, Na+, K+ Fe2+, SO42−, Clˉ and F− to evaluate the hydrochemical behaviour with SSP (sodium soluble percentage), SAR (sodium absorption ratio), % Na (percentage sodium), RSC (residual sodium carbonate), KR (Kelly’s ratio), PI (permeability index) and MH (magnesium hazards). These positive and negative ions have been further correlated with the maximum annual rainfall within the study area to find out the variations between these ions for the precipitation. Suitability of groundwater for drinking purposes around the catchment was not suitable except in a few places. Irrigational suitability of groundwater showed that the water is within the limit for irrigation except in a few locations. Wilcox diagram depicts that 90% of the pre-monsoon samples and 65% of the post-monsoon samples fell into excellent to good category zone. US salinity diagram explains that 71% of pre-monsoon samples belong to medium-salinity-hazard to low-sodium-content zones, whereas 50% of post-monsoon samples fall into high-salinity-hazard to low-sodium-content zone. Gibbs’s plot showed that the water–rock processes control the geochemistry of the Aland region in both monsoon seasons. Chadha’s diagram depicts that 56.25% of the groundwater samples fall under the subfield of Ca2+–Mg2+–Cl− water type with permanent hardness during pre-monsoon season, whereas 50% of groundwater samples falls under the subfield of Ca2+–Mg2+–HCO3− water type with temporary hardness during post-monsoon season.
Similar content being viewed by others
Introduction
The quality of water is an essential factor for controlling water management studies. Generally, anthropogenic and geogenic activities are responsible for groundwater contamination. Industrial, agricultural and domestic wastes, rainfall pattern and infiltration rate are also responsible for the deterioration of groundwater quality. Once a groundwater system is contaminated, it continues for many years because of the very slow movement of water in them. It may become challenging to monitor their quality by removing the pollutants from the contaminated aquifer (Devi et al. 2009). The degrading groundwater quality has become a severe universal issue for sustainable development (Adimalla and Jianhua 2019; Li et al. 2019), and therefore, it is very important to understand the hydrogeochemical characteristics of groundwater for sustainable resource development and governance (Huzefa et al. 2020). Subsurface water is mostly limited due to the scanty precipitation, which leads to high evaporation and runoff, especially in arid and semi-arid regions (Kadam et al. 2020; Camacho Suarez et al. 2015). Almost one-tenth of worldwide diseases occur due to polluted water and poor sanitation (Fewtrell et al. 2007) and there are nearly 2.5 billion of the population without access to clean water (WHO 2011).
The quality of groundwater is controlled by geology, recharged water, water–soil interaction, recharge and discharge conditions of the area, soil–gas interaction and reactions occurring in the aquifer (Biswal and Kalita 2009; Selvakumar et al. 2014). It also plays a significant role in agricultural production and human health condition (Mohammed-Aslam et al. 2016). Polluted drinking water naturally affects the soil–crop–water system. If the deterioration of water quality is not checked at the right time, it will not be suitable for many purposes (Mohammed-Aslam et al. 2016). Geochemical studies generally deal with chemical parameters and its portability for drinking, irrigation and industrial purpose. Sodium absorption ratio (SAR), high salinity and percentage sodium (%Na) play a significant role in the ranking of quality of groundwater for irrigational purposes. Evaluation of hydrochemical quality of groundwater reveals the interaction groundwater with the surrounding rocks and minerals (Gibbs 1970). The demand of freshwater resources has been increased in the Aland region mainly due to the population growth and severe agricultural activities. Moreover, the industrial establishments are attracted in this region, due to special schemes in Hyderabad–Karnataka region, wherein this area is located. Therefore, it has become essential to manage the groundwater deployment in the area. The goals of this study are to evaluate the hydrochemical behaviour and represent the appropriate picture of present water quality in the study region through SSP, SAR, RSC, KR, PI, MH and %Na values with irrigational and domestic suitability of water quality using US salinity diagram, Wilcox diagram, Gibb’s plot and Chadha’s plot.
Study area
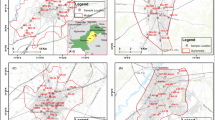
Amarja Reservoir Catchment comes under Aland Taluk of Northern Karnataka region. The region is well known for water scarcity. It covers an area of 544.76 sq. km and surrounded between latitude 17º50′-9″-N to 17º72′´-20″-N and longitude 76º45′-8″-E to 76º55′-20″-E (Fig. 1). The study area comes under semi-arid provinces consisting mainly of basalt with intertrappean beds at some places (Rizvi and Mohammed-Aslam 2018, 2019). The region also contains deep black soils that have been derived from Deccan traps (Rizvi and Mohammed-Aslam 2018, 2019). The summer season is from February and June. The south-west monsoon is from June to September. The month of December comes under the coldest month, having a temperature variation 29.5 °C to and 10 °C (CGWB 2012). In the peak of summer, the temperature was found raised to 45 °C. The relative humidity is about 26% in the summer season and increases by up to 62% in the winter period (CGWB 2012). The area receives an average annual rainfall of about 777 mm, while the minimum yearly rainfall is 342 mm and the maximum yearly rainfall is 1270 mm (CGWB 2012).
Data collection and methodology
A total number of 32 samples were collected from the bore wells and open wells in the study area (Fig. 1). Sampling has been done in both pre-monsoon (April 2016) and post-monsoon (November 2016) seasons. Drinking water samples were collected with high precautions, ensuring that there is no contamination takes place during the time of sampling. Intensive care has been taken during the collection of the water sample from freshwater resources in one-litre bottles. Geographical coordinates and elevation at each location were recorded by using GPS (global positioning system). ArcGIS 10.3 software has been used in the study for preparing the geospatial maps. The geospatial maps of physicochemical parameters were prepared to analyse the distribution pattern within Amarja reservoir catchment by using this software. TDS, pH and EC test were done at the field itself by using TDS, pH and electrical conductivity meters. The analyses of the rest of the parameters were performed in the laboratory within 24 h after the collection of the samples. Magnesium, calcium, sodium, potassium, chloride, fluoride, alkalinity, nitrate, sulphate and total hardness have been analysed for the water samples. The analysis was done based on BIS standards (2012).
Result and discussion
Geochemical characterisation has been evaluated based on sodium soluble percentage, sodium absorption ratio, residual sodium carbonate, percentage sodium, permeability index, Kelly’s ratio and magnesium hazards. US salinity diagram was used to categorised salinity hazard zone, whereas water–rock interaction was done based on Gibb’s diagram. Chadha’s plot was used to understand the hydrochemical facies of groundwater within the study region. The details of the analysed results are shown in Tables 1 and 2, respectively.
Groundwater suitability for drinking purposes
pH
The pH of analysed samples varies from 7.16 to 8.88 in the pre-monsoon season, whereas it ranged from 7.30 to 8.75 during the post-monsoon season. The spatial distribution map of pH of both seasons is shown in Figs. 2 and 3. According to the BIS guideline (2012), the permissible limit of pH is in the range from 6.5 to 8.5 and 9.38% of the samples during pre-monsoon, whereas 3.12% during the post-monsoon season were exceeded the maximum permissible limit. Though pH has no direct effect on human health, all biogeochemical reactions are sensitive to the variation of pH (Subba Rao and Krishna Rao 1991).
Electrical conductivity
Electrical conductivity values in the study area range from 168 to 1479 µS/cm in the pre-monsoon season and it varies from 181 to 1100 µS/cm during post-monsoon. 40.63% of the samples have shown conductivity values less than 500 µS/cm, 50% were in between 500 and 1000 µS/cm, whereas 9.37% of the groundwater samples have conductivity value more than 1000 µS/cm. Higher amounts of electrical conductivity depend upon temperature, concentration and type of ions present in groundwater (Adimalla and Venkatayogi 2018). Geospatial mapping of the conductivity of both monsoon seasons is shown in Figs. 4 and 5.
Total dissolved solids (TDS)
Higher concentrations of dissolved solids increase the density of water and influence the osmoregulation of freshwater organism (Sharath et al. 2018). The lower concentration of TDS in groundwater is due to the repeated dilution through rainfall and influent nature of surface water bodies (Mohammad Muqtada et al. 2018). TDS values of pre-monsoon season were found in the range from 108 to 947 mg/L and showed the variation from 116 to 704 mg/L during post-monsoon. The acceptable limit of TDS in groundwater is 500 mg/L as per BIS 2012. Only 21.88% of the groundwater samples were exceeded the permissible limit during pre-monsoon season, whereas 34.38% of the samples were above the limit during post-monsoon season. The spatial distribution map of TDS of both the seasons is shown in Figs. 6 and 7. The variation in TDS values may also occur due to Geogenic activities in the region.
Calcium and magnesium
Calcium in the groundwater of the study area varies from 16 to 368.16 mg/L in the pre-monsoon season, whereas 22.4–201.6 mg/L was recorded during post-monsoon season. Only 9.37% of samples were exceeded the permissible limit in the pre-monsoon season, whereas 3.12% were above during post-monsoon season. Spatial distribution map of calcium of both seasons is shown in Figs. 8 and 9. The presence of a large amount of carbon dioxide may increase the solubility of calcium up to 200–300 mg/L with an availability of the bicarbonate (Hem 1985). Magnesium in the groundwater of the study area is varying from 0 to 92.15 mg/L during pre-monsoon season, whereas, during the post-monsoon season, magnesium values showed variation from 1.01 to 201.6 mg/L. Spatial distribution map of both monsoon seasons is depicted in Figs. 10 and 11. During pre-monsoon season, 31.25% of groundwater sample of the study area has magnesium concentration above the permissible limit, whereas 50% of the samples were exceeded during post-monsoon season.
Sodium and potassium
The concentration of sodium of pre-monsoon seasons was found in the range from 7.89 to 13.76 mg/L, whereas it was in a range from 7.50 to 13.50 mg/L during post-monsoon season. According to the BIS (2012) guidelines, the maximum permissible limit is 200 mg/L, and all the samples were in the allowable limit during both monsoon seasons. Gceospatial map of both monsoon seasons is shown in Figs. 12 and 13. A higher concentration of sodium in groundwater makes it unsuitable for domestic use and causes severe health problems such as hypertension and kidney disorders in the human body (Adimalla and Venkatayogi 2017). Potassium concentration in groundwater ranges from 14.5 to 20.02 mg/L during pre-monsoon season, whereas it varies from 10.5 to 19.84 mg/L during post-monsoon season. All the groundwater samples were in the permissible limit. Geospatial distribution map for both the seasons is depicted in Figs. 14 and 15.
Iron
The water with no iron concentration is not considered as healthy because iron always plays a significant role in the nutrition of our body (Sharath et al. 2018). Iron value of pre-monsoon season was found in the range from 0.002 to 0.08 mg/L, whereas the values were found varying from 0.001 to 0.08 mg/L during post-monsoon season. The acceptable permissible limit is 0.3 mg/L as per BIS standard (2012). None of the samples was exceeded to the allowable limit in both monsoon periods. The occurrence of iron strongly depends on the oxidation intensity of medium where it occurs (Hem 1985). Spatial distribution map of iron of both the seasons is shown in Figs. 16 and 17 for better understanding.
Alkalinity (carbonate and bicarbonate)
Carbonate in the groundwater of the study area varies from 0 to 122.4 mg/L during pre-monsoon, whereas it varies from 0 to 12 mg/L during post-monsoon season. The geospatial distribution map of both the seasons is shown in Figs. 18 and 19. Bicarbonate varies from 7.32 to 622.2 mg/L, in the pre-monsoon, whereas it varies from 43.92 to 326.96 mg/L during post-monsoon. Spatial distribution map of bicarbonate concentrations in groundwater is shown in Figs. 20 and 21. All the carbonate value was in the permissible range, but 18.75% of groundwater sample of bicarbonate has higher concentration during pre-monsoon season, whereas 50% were above the allowable limit during post-monsoon season. Geogenic activity may be responsible for the higher concentrations of bicarbonate within the region.
Chloride and fluoride
The chloride values of pre-monsoon season were found in the range from 32.62 to 373.98 mg/L. The permissible limit of chloride in potable water is 250 mg/L (BIS 2012) and only 6.25% of groundwater samples were exceeded the allowable limit during pre-monsoon season. In the post-monsoon season, it varies from 34.03 to 300.62 mg/L and 6.25% of the samples were above the concentration. Leaching of upper soil layers and dry climate may be responsible for the higher concentrations of chloride (Bhardwaj and Singh 2011). Geospatial mapping of chloride of both seasons is shown in Figs. 22 and 23.
The fluoride concentration in groundwater shows a range of 0.65 to 1.97 mg/L. The highest value of fluoride was recorded in Nagelagaon (1.97 mg/L), whereas the lowest value was noted in Tadola village. In the pre-monsoon season, 78.13% of the groundwater samples of the region were above the desirable limit (1.00 mg/L), whereas 15.62% of the groundwater has more than 1.5 mg/L of fluoride which is the maximum permissible limit for drinking purposes as per BIS standard (2012). During post-monsoon season, the fluoride values varied from 0.76 to 1.54 mg/L. The highest value of fluoride was recorded in Padsawli (1.54 mg/L), whereas the lowest value was in Korahalli village. Spatial distribution pattern of fluoride of both the seasons is depicted in Figs. 24 and 25. 90.63% of the groundwater samples of the study area showed a higher concentration of fluoride above the desirable limit (1.00 mg/L). In contrast, only 3.12% of the groundwater was above the maximum permissible (1.5 mg/L) limit of fluoride for drinking purposes. The sources of fluoride in the groundwater of the study region is mainly coming from the geological occurrence. The deep circulation of fluoride-rich groundwater between the lateralised basalts could be responsible for the event of fluoride in both the shallow and deeper aquifers (Raymond and Uday 2011). Excess fluoride concentration in groundwater can cause dental and skeletal fluorosis.
Nitrate and sulphate
Nitrate varies from 12 to 96 mg/L in pre-monsoon, whereas it ranged from 16 to 77 mg/L during post-monsoon. It has been noticed that 21.87% of the groundwater sample contains excess nitrate during pre-monsoon season, whereas 65.62% of the samples were above the permissible limit (> 45 mg/L) in the study area, which is not suitable for drinking purpose. Geospatial distribution map has been prepared for both the monsoon periods for the better understanding of excess nitrate within the region, and it is depicted in Figs. 26 and 27. A higher concentration of nitrate generally occur due to the inappropriate well structure, excessive use of chemical fertiliser or improper disposal of human, and animal waste and the higher concentration of nitrate in groundwater can lead to cancer (Dissanayake et al. 1987).
Sulphide is derived from the reduction of sulphate compounds, dissolved under low-oxygen condition when anaerobic bacteria are present (Lehr et al. 1980). The sulphate values of pre-monsoon seasons were found in the range from 7.2 to 128.65 mg/L, whereas, during the post-monsoon season, it varies from 14.48 to 87.33 mg/L. According to the BIS standard (2012), the permissible limit of sulphate in potable water is 200 mg/L, and all the samples of the study area were in permissible limit during both the monsoon seasons. Spatial distribution map of sulphate of both seasons is shown in Figs. 28 and 29.
Total hardness
The hardness of water can be caused by the presence of multivalent metallic cations (Hem 1985). The hardness of water has a significant effect on pH and always substantial in the stability control of pH. The hardness values of pre-monsoon season were found in the range from 20 to 704 mg/L and 32 to 398 mg/L during post-monsoon season. Geospatial distribution of hardness of both seasons is shown in Figs. 30 and 31. Nearly 56.25% of the groundwater samples were exceeded the permissible limit (BIS 2012) during pre-monsoon season, whereas 62.5% of the samples were found to be above the allowable limit (> 200 mg/L) during post-monsoon season. The higher concentrations of the hardness may occur due to the weathering of calcium bearing minerals or disproportionate use of lime to the soil in agricultural regions.
Table 3 illustrates the percentage-wise drinking water suitability of study area.
Hydrochemical facies for groundwater (Chaddha’s diagram)
Hydrochemical facies of a particular region are always associated with the geology of that region, and the distribution of the facies is influenced by hydrogeological controls (Nagaraju et al. 2016). Hydrochemical facies can be well classified based on Chadha’s diagram (Chadha 1999). Chadha’s diagram explains the differences in milliequivalent percentage between alkaline earth’s (calcium + magnesium) and alkali metals (sodium + potassium) expressed as percentage reacting value is plotted on the X-axis. The difference in milliequivalent percentage between weak acidic anions (carbonate + bicarbonate) and strong acidic anions (chloride + sulphate) is plotted on the Y-axis. The square or rectangular field depicts the overall character of the water (Nagaraju et al. 2016). The result of the chemical analyses data of all the samples was plotted on Chadha’s diagram. The Chadha’s plot of pre-monsoon season depicts that 56.25% of the groundwater samples fall under the subfield of Ca2+–Mg2+–Cl− water type with permanent hardness. The remaining 43.75% of samples fall under the subfield of Ca2+–Mg2+–HCO3− water type with temporary hardness (Fig. 32). In the post-monsoon season, the plot depicts that 50% samples fall under the field of Ca2+–Mg2+–HCO3− water type with temporary hardness, whereas the remaining 50% samples belong to the subfield of Ca2+–Mg2+–Cl− water type with permanent hardness (Fig. 33).
Gibbs’s plot
Gibb’s plot is used to characterise the quality of water to precipitation, evaporation/crystallisation and rock–water interaction. In 1970, Gibb’s has established a relation water chemistry and aquifers lithology. In Gibb’s plot, TDS on y-axis, Na++K+/Na++K++Ca2+ on x-axis for cations and Cl−/Cl−+HCO3− on x-axis for anion are plotted, respectively. It is useful to differentiate the interaction of groundwater through precipitation or rock evaporation. Gibb’s has recognised three kinds of fields which are known as precipitation, evaporation/crystallisation and rock–water interaction. Weathering dominated water would be having higher Ca2+ and HCO3− concentration, whereas evaporation/crystallisation-controlled water would be having a higher concentration of Na+ and Cl− on Gibb’s plot. Equations (1) and (2) describe Gibb’s ratio for cation and anion, respectively.
where Na, K, Ca, Cl and HCO3 are expressed in meq/l.
Gibb’s plot of both the seasons of different cations and anions is shown in Figs. 34 and 35, respectively. Rock dominance controls are seen for groundwater for both seasons.
Irrigational suitability of groundwater
The irrigational suitability of groundwater can be evaluated through the indices for salinity, chlorinity and sodicity. SAR, RSC, %Na, Kelly’s ratio, magnesium hazards, permeability index are the most critical indices in the estimating of groundwater for irrigational suitability. The higher concentration of sodium always affects the suitability for irrigation on the soil in respect of exchangeable calcium and magnesium ions. It can be evaluated by sodium soluble percentage (SSP), sodium absorption ratio (SAR), residual sodium carbonate (RSC) and %Na indices.
Soluble sodium percentage (SSP)
Sodium in water replaces calcium in the soil through the base exchange process, which is responsible for decreasing the soil permeability (Carroll 1962). Soluble sodium percentage (SSP) values less than or equal to 50 are considered as good quality for irrigation purpose. SSP values greater than 50 are unacceptable for irrigation as the permeability is very low in this case (Table 4). 100% of the samples were in the good quality class of SSP values in both the seasons. SSP can be evaluated by using Eq. (3).
where Na, Ca, Mg and Na are expressed in meq/l.
Sodium absorption ratio (SAR)
SAR values less than 10 would be suitable for irrigation, whereas greater than 10 would be not comfortable for irrigational purposes (Richards 1954). 100% of groundwater samples of both seasons fall under the excellent class of SAR values (Table 4). SAR can be evaluated by using Eq. (4).
where Na, Ca and Mg are expressed in meq/l.
Residual sodium carbonate (RSC)
A higher concentration of carbonate causes calcium and magnesium to precipitate as bicarbonate in water (Ravikumar et al. 2011). The hazardous effect of carbonate and bicarbonate in water for agricultural purposes can be determined by residual sodium carbonate (RSC) analysis (Eaton 1950). According to RSC values (Table 4), 90.62% of groundwater samples belong to the safe category, whereas 9.38% of samples fall under marginal zone during the pre-monsoon season. In the post-monsoon season, 100% of the samples were categorised as a safe class of RSC values. A negative value of RSC indicates the higher concentrations of Ca and Mg in groundwater (Adimalla 2019). RSC can be evaluated (Eaton 1950) by using Eq. (5).
where HCO3, CO3, Ca and Mg are expressed in meq/l.
Percent sodium (%Na)
Percent sodium (%Na) is also an important parameter to study sodium hazards. Soil permeability is reduced due to sodium (Joshi et al. 2009). According to the %Na values (Table 4), 75% of samples were grouped into excellent class, 18.75% were in good, and 6.25% were in permissible classes of percentage sodium during pre-monsoon season. In the post-monsoon season, 90.62% of the samples were fallen into excellent, 6.25% samples in the good class, whereas 3.13% of the sample falls under permissible classes of %Na values. %Na can be evaluated (Wilcox 1955) by using Eq. (6).
where Na, K, Ca and Mg are expressed in meq/l.
Wilcox diagram for both seasons is shown in Figs. 36 and 37, respectively.
Kelly’s ratio (KR)
Kelly’s ratio (KR) is used for determining the sodium hazards on water quality for irrigational purpose (Kelly 1940). Table 4 represents the suitability classification of water for different seasons in the study area based on Kelly’s ratio. 100% of the groundwater samples of both the seasons fell into the suitable class of Kelly’s ratio values. Kelly’s ratio can be evaluated by using Eq. (7).
where Na, Ca and Mg are expressed in meq/l.
Permeability index (PI)
Soil permeability is affected due to through continuous use of water for irrigation as precipitation of certain elements in soil reduces the void space depending upon the quality, thereby hampering the water dynamics (Doneen 1964). Doneen proposed permeability index (PI) for characterising the suitability of irrigational water. It has been categorised into three classes. Class I (> 75%) and Class II (25–75%) represents the waters suitable for irrigation with 75% or more of maximum permeability. In contrast, Class III (< 25%) characterises the water unsuitable for irrigation with 25% of maximum permeability (Table 4). During pre-monsoon season, 18.75% of the samples enter to the Class III and unfit for irrigation purposes, whereas 68.75% samples belong to Class II and 12.5% fall under the Class I, shows suitability for irrigation purposes with maximum permeability. In the post-monsoon seasons, 34.37% of the samples belong to Class III, whereas 62.5% enter to Class II and 3.13% of the samples enter to the Class I. Permeability index can be evaluated by using Eq. (8).
where Na, HCO3, Ca and Mg are expressed in meq/l.
Magnesium hazards (MH)
Magnesium and calcium usually maintain a state of equilibrium in groundwater (Adimalla and Venkatayogi 2018). A higher concentration of magnesium in water can affects the soil quality through changing it into alkaline nature. During pre-monsoon season, 78.13% of the samples belong to the suitable class of irrigation according to MH values (Table 4), whereas 21.87% of the samples were categorised as unsuitable. 65.62% of the samples were grouped as a suitable class of irrigation, and 34.38% were belonging to the unsuitable class during post-monsoon seasons. Magnesium hazard ratio can be evaluated (Szabolcs and Darab 1964) by using Eq. (9).
where Mg and Ca are expressed in meq/l.
Classification of irrigational suitability through US salinity diagram
The electrical conductivity represents salinity hazards, whereas the sodium hazards are described in terms of sodium absorption ratio (SAR) on the USSL chart (USSL 1954). Excess sodium in water produces the undesirable effects of changing soil properties and reducing soil permeability (Adimalla and Venkatayogi 2018). Decreasing content of ions such as magnesium and calcium are often attributed with an increase in sodium which may result in high SAR values (Mohammad Muqtada et al. 2018). In the pre-monsoon season, 6.25% of groundwater samples fall into C1–S1 group of USSL diagram, which represents low-salinity and low-sodium hazard zone, and this water is most suitable for irrigation purposes. 65.63% of samples belong to the category of C2–S1 which indicates medium-salinity hazards with low-alkaline water and this can be used for irrigation purposes with little impact of exchangeable sodium (Adimalla and Venkatayogi 2018). 28.12% samples fall into C3–S1 class which stand for high-salinity and low-alkaline water, and this type of water needs extensive treatment before applied to the soil irrigation due to high salinity (Adimalla 2019). During post-monsoon season, 6.25% samples fall into C1–S1 class, 40.62% samples belong to C2–S1, and 53.13% samples fall into the category of C3–S1 (Figs. 38, 39). High levels of sodium ion in groundwater with relatively low levels of calcium ion are known to affect the subsurface structure, and degree of water is infiltrating the strata (Sadashivaiah et al. 2008). The summary of groundwater quality for irrigational purposes in both monsoon seasons is given in Tables 5 and 6, respectively.
Domestic suitability using hardness
Domestic suitability of groundwater can be evaluated based on hardness of water (Durfor and Becker 1964). Table 7 represents the domestic suitability classification of water of different seasons based on hardness values in mg/L. In both monsoon seasons, 96.88% of groundwater samples fall into the soft water class, whereas 3.12% samples belong to moderately to hard water zone. Domestic suitability of water using hardness can be calculated (Durfor and Becker 1964) using Eq. (10).
where Ca, Mg and HCO3 are expressed in mg/L.
Industrial suitability
The industrial establishments are attracted in this region, due to special schemes in Hyderabad-Karnataka Region, wherein this area is located. Therefore, industrial Suitability has been done to check whether the samples are fit for the industrial suitability or not. The corrosivity ratio has been used to evaluate industrial usefulness (Table 7). Corrosion represents an electrolytic process through which metal surfaces are corroded (Devi et al. 2009). The result shows that 78.13% of samples were of suitable class (< 1), whereas 21.87% of samples were unsuitable (> 1) during pre-monsoon season. In the post-monsoon season, only 28.12% of groundwater samples fell into a suitable class, whereas 71.88% were into an unsuitable category. Corrosivity ratio can be evaluated (Badrinath et al. 1984) using Eq. (11).
where Cl, SO4, CO3 and HCO3 are expressed in mg/L.
Collin’s ratio
Collin’s ratio (CR) is used to check the suitability of water for drinking purposes. Water is suitable for drinking purposes if CR < 1. Water is considered as contaminated with saline water if the values of CR between 1 and 3 and injurious to health if CR > 6. 62.5% of samples belong to the suitable class, whereas 37.5% samples were contaminated with saline water in the pre-monsoon season (Table 7). This may result due to the excess fertiliser activity within the region. In the post-monsoon season, 71.88% samples fall into a suitable class, whereas 25% of groundwater samples were contaminated with saline water and 3.12% fall into the healthy risk zone. Collin’s ratio (proposed by Simpson 1946) of water can be calculated by using Eq. (12).
where Cl, CO3 and HCO3 are expressed in meq/l.
Domestic and industrial suitability of groundwater of different monsoons is given in Tables 8 and 9, respectively.
Rainfall water quality linkage
An attempt has been made to correlate the hydrochemical parameters with the rainfall. In this concern, maximum annual rainfall data in the study area of 2016 were collected from the Karnataka State Natural Disaster Monitoring Centre (KSNDMC) website. The maximum rainfall in Aland was noted in July (108 mm) and September (107 mm), whereas the minimum rain was recorded in January (4.5 mm). Although the region has come under a semi-arid zone, it is also well known for scanty rainfall. Correlation analysis has been done to check the variations of positive and negative ions to the rainfall. Figure 40 depicts the maximum annual rainfall in the study area. The relationship between rainfall, physical parameters, positive and negative ions of all the location is shown in Figs. 41, 42 and 43, respectively. The result indicates that the rainfall within the study area does not influence water quality. The main reason could be the texture and type of soil presents in the region. The result is further confirmed by the Gibbs’s plot of both seasons where it is clearly shown that rock dominance factor is controlling the groundwater chemistry and there is no effect of precipitation. Calcium and magnesium concentrations in groundwater play a significant role in the estimation of groundwater quality, and Mg can give more adverse effects on soils than calcium (Adimalla 2019). Runoff pattern can also influence the water quality.
Conclusion
Drinking water suitability of groundwater around the catchment was not good except at few places. The pH of groundwater was close to the permissible limit in both monsoon seasons with exceeding at some locations. A higher concentration of TDS, Ca, Mg, HCO3, Cl, NO3 and hardness were recorded except in few cases. At most of the locations, fluoride concentrations were close to the permissible limit with exceeding few places during pre-monsoon and post-monsoon seasons. Fluoride-affected areas in both the monsoon seasons were noticed as Telikuni (1.48 mg/L) village, Kinnisultan (1.49 mg/L), Aland (1.50 mg/L), Jidga (1.51 mg/L), Padsawli (1.54 mg/L), Nagelagaon (1.97 mg/L), Sakkarga (1.64 mg/L), Kamanalli (1.58 mg/L), Hebli (1.49 mg/L), Khairat 91.49 mg/L) and Jheerahalli (1.46 mg/L) village. The highest concentration of fluoride was recorded in Nagelagaon (1.97 mg/L) during pre-monsoon and Padsawli village (1.54 mg/L) during post-monsoon. Environmental awareness programme for the health implications of fluoride in the study area may be conducted from time to time by the stakeholders concerned. Highest nitrate concentration was recorded in Kamanalli (96 mg/L) village during pre-monsoon, whereas Jamga Khandala (77 mg/L) village during post-monsoon.
Irrigational suitability of groundwater showed that the water is within the limit for irrigation except in a few locations. The USSL diagram illustrated the salinity hazards and suggested the need for unique soil management before use. Gibb’s plot indicates that groundwater chemistry is controlled by rock dominance factor for both seasons. Geochemical classification of groundwater using Chadha’s diagram is indicating Ca2+–Mg2+–Cl− and Ca2+–Mg2+–HCO3− water type for both seasons. Correlation analysis showed that water quality within the region is not influenced by rainfall which is further confirmed by Gibb’s plot. Overall, the water quality analysis revealed that the groundwater quality of catchment was not good for the public health point of view. Therefore, community awareness programme and steady survey through municipalities’ is needed for a safeguard public health. The lower part of the catchment requires proper management for groundwater before utilising for domestic purposes. GIS-based spatial distribution maps are useful in the better understanding of mapping the vulnerable zones of the catchment for adopting a better management strategy.
References
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Health 11:109–123. https://doi.org/10.1007/s12403-018-0288-8
Adimalla N, Jianhua Wu (2019) Groundwater quality and associated health risks in a semi-arid region of south India: implication to sustainable groundwater management. Hum Ecol Risk Assess 25(1–2):191–216. https://doi.org/10.1080/10807039.2018.1546550
Adimalla N, Venkatayogi S (2017) Contamination of Fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet, Telangana State, India. Appl Water Sci 7:2501–2512. https://doi.org/10.1007/s13201-016-0441-0
Adimalla N, Venkatayogi S (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangna State, South India. Appl Water Sci 8:44. https://doi.org/10.1007/s13201-018-0682-1
Badrinath SD, Raman A, Gadkari SK, Mhaisalkar VA, Despande VP (1984) Evaluation of Carbonate Stability indices for Sabarmati river water. Indian Water Assess 16:163–168
Bhardwaj V, Singh DS (2011) Surface and groundwater quality characterisation of Deoria District, Ganga Plain, India. Environ Earth Sci 63:383–395
BIS (2012). Indian Standards Specifications for drinking water. IS:10500 2nd Revision Bureau of Indian Standards (BIS), New Delhi
Biswal AK, Kalita P (2009) Ground water scenario and its management in Shillong Urban Agglomerate, Meghalaya. In: Ramanathan AL, Bhattacharya P, Keshari AK, Bundschuh J, Chandrasekharam D, Singh SK (eds) Assessment of ground water resources and management. I K International Publishing House Pvt Ltd, New Delhi, India, pp 1–8
Camacho Suarez VV, Saraiva Okello AML, Wenninger JW, Uhlenbrook S (2015) Understanding runoff processes in a semi-arid environment through isotope and hydrochemical hydrograph separations. Hydrol Earth Syst Sci 19(10):4183–4199
Carroll D (1962) Rainwater as a chemical agent of geologic processes: a review. USGS Water Supply Paper, p 1535. https://pubs.usgs.gov/wsp/1535g/report.pdf
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Devi OJ, Belagali SL, Balasubramanian A, Bose S, Senthilkumar G, Ramaswamy SN, Ramanathan AL (2009) Evaluation of Groundwater quality for irrigation and drinking purposes in selected taluks of Dakshina Kannada district in Karnataka, India. International Society of Groundwater for Sustainable Development, Special Publication, pp 301–318
Dissanayake CB, Niwas JM, Weerasooriya SVR (1987) Heavy metal pollution of the mid canal of Kandis; An environmental case study from Sri Lanka. Environ Res 42:24–35
Doneen LD (1964) Notes on water quality in agriculture. Published as a water science and engineering paper, 4001. Department of water science and engineering, University of California
Durfor CN, Becker E (1964) Public water supplies of the 100 largest cities in the United States. Geological survey water supply, vol 1812. U.S. Government Printing Office, Washington, p 364
Eaton FM (1950) Significance of carbonate in irrigation water. Soil Sci 69:123–133
Fewtrell L, Prüss-Ustün A, Bos R, Gore F, Bartram J (2007) Water, sanitation and hygiene: quantifying the health impact at national and local levels in countries with incomplete water supply and sanitation coverage. World Health Organization, Geneva
Gibbs RJ (1970) Mechanisms controlling worlds water chemistry. Science 170:1088–1090
Groundwater Information Booklet, Gulbarga District, Karnataka (2012) South-western Region. Bangalore. http://cgwb.gov.in/District_Profile/karnataka/2012/Gulbarga-2012.pdf
Handa BK (1969) Description and classification of media for hydro-geochemical investigations. In: Symposium on ground water studies in arid and semiarid regions, Roorkee
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 2nd edn. UG Geol Surv Water Supply Paper 2254:363
Huzefa S, Himanshu G, Ajaykumar K, Bhavana U (2020) Hydrochemical characterisation of groundwater from semi-arid region of western India for drinking and agricultural purposes with special reference to water quality index and potential health risks assessment. Appl Water Sci 10:204. https://doi.org/10.1007/s13201-020-01287-z
Joshi DM, Kumar A, Agrawal N (2009) Studies on physicochemical parameters to assess the water quality of river Ganga for drinking purposes in Hardiwar district. Rasayan J Chem 2(1):195–203
Kadam AK, Umrikar BN, Sankhua RN (2020) Assessment of recharge potential zones for groundwater development and management using geospatial and MCDA technologies in a semi-arid region of Western India. SN Appl Sci 2(2):312. https://doi.org/10.1007/s42452-020-2079-7
Kelly WP (1940) Permissible composition and concentration of irrigation waters. Proc Am Soc Civ Eng 66:607–613
Lehr JH, Gass TE, Pettyjohn WA, DeMarre J (1980) Domestic Water Treatment. McGraw-Hill Book Company, New York
Li P, He X, Guo W (2019) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an City on the Loess Plateau of northwest China. Hum Ecol Risk Assess Int J 25(1–2):11–31
Mohammad Muqtada AK, Kishan Raj PM, Aina M, Zakiyah AK, Hafzan EM (2018) Evaluating the suitability of shallow aquifer for irrigational purposes in some parts of Kelantan, Malaysia. Bull Geol Soc Malays 66:57–64
Mohammed-Aslam MA, Lakshmanadinesh K, Nallusamy B, Bharathkumar L, Asokan A (2016) Hydrogeochemical characteristics and classification of groundwater of Chikballapur Taluk, Karnataka. Environ Geochem 19(1–2):25–30
Nagaraju A, Muralidhar P, Sreedhar Y (2016) Hydrogeochemistry and groundwater quality assessment of Rapur Area, Andhra Pradesh, South India. J Geosci Environ Prot 4:88–99. https://doi.org/10.4236/gep.2016.44012
Ravikumar P, Venkatesharaju K, Prakash KL, Somashekar RK (2011) Geochemistry of groundwater and groundwater prospects evaluation, Anekal Taluk, Bangalore urban district, Karnataka, India. Environ Monit Assess 193:93–112
Raymond AD, Uday P (2011) Occurrence of fluoride in the drinking water sources from Gad River Basin, Maharashtra. J Geol Soc India 77:167–174
Richards LA (1954) Diagnosis and improvement of saline-alkali soils, Agriculture, handbook, vol 150. US Department of Agriculture, Washington, p 60
Rizvi SS, Aslam M (2018) Estimation of hypsometric integral and groundwater potential zones of Amarja Reservoir Catchment, Karnataka, India using SRTM data and geospatial tools. Int J Econ Environ Geol 9(2):75–85
Rizvi SS, Mohammed A (2019) Microbiological quality of drinking water in Amarja Reservoir Catchment, Aland Taluk, Karnataka, India. Curr Sci 117(1):114–121. https://doi.org/10.18520/cs/v117/i1/114-121
Sadashivaiah C, Ramakrishnaiah CR, Ranganna G (2008) Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State, India. Int J Environ Res Public Health 5(3):158–164
Selvakumar S, Ramkumar K, Chandrasekar N, Magesh NS, Kaliraj S (2014) Groundwater quality and its suitability for drinking and irrigational use in the Southern Tiruchirappalli District, Tamil Nadu, India. Appl Water Sci 7(1):411–420
Sharath RB, Channabasappa K, Sethi C, Mohammed-Aslam MA (2018) Physical–chemical characterisation and water quality index (WQI) assessment of Bhusnoor Area, Kalaburagi District, Karnataka. J Appl Geochem 20(4):474–481
Simpson TR (1946) Saline Basin Investigation Bull Calif. Div. Water Resource, Sacramento, 52:230
Subba Rao N, Krishna Rao G (1991) Groundwater quality in Vishakhapatnam urban area, Andhra Pradesh. Indian J Environ Health 33(1):25–30
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, Trans, vol II, pp 803–812
US Salinity Laboratory (1954) Diagnosis and improvement of salinity and alkaline soil. USA Hand Book no. 60, Wasington DC
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Circular 969, Washington
World Health Organization (WHO) (2011) Valuing water, valuing livelihoods: guidance on social cost-benefit analysis of drinking-water interventions, with special reference to small community water supplies. In: Cameron John, Hunter Paul, Jagals Paul, Pond Katherine (eds) London. England, IWA
Acknowledgements
The authors would like to express his special thanks to the Enrico Driol, Editor-in-Chief, for his quick response. The authors further extend their gratitude to the reviewers for their valuable comment and suggestions.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammed-Aslam, M.A., Rizvi, S.S. Hydrogeochemical characterisation and appraisal of groundwater suitability for domestic and irrigational purposes in a semi-arid region, Karnataka state, India. Appl Water Sci 10, 237 (2020). https://doi.org/10.1007/s13201-020-01320-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01320-1