Abstract
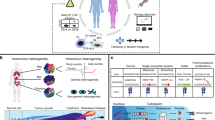
Glioblastoma tumors exhibit extensive inter- and intratumoral heterogeneity, which has contributed to the poor outcomes of numerous clinical trials and continues to complicate the development of effective therapeutic strategies. Most in vitro models do not preserve the cellular and mutational diversity of parent tumors and often require a lengthy generation time with variable efficiency. Here, we describe detailed procedures for generating glioblastoma organoids (GBOs) from surgically resected patient tumor tissue using a chemically defined medium without cell dissociation. By preserving cell-cell interactions and minimizing clonal selection, GBOs maintain the cellular heterogeneity of parent tumors. We include details of how to passage and cryopreserve GBOs for continued use, biobanking and long-term recovery. In addition, we describe procedures for investigating patient-specific responses to immunotherapies by co-culturing GBOs with chimeric antigen receptor (CAR) T cells. It takes ~2–4 weeks to generate GBOs and 5–7 d to perform CAR T cell co-culture using this protocol. Competence with human cell culture, tissue processing, immunohistology and microscopy is required for optimal results.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Kopper, O. et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 25, 838–849 (2019).
Lee, S. H. et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173, 515–528.e17 (2018).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e10 (2018).
Yan, H. H. N. et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 23, 882–897.e11 (2018).
Yao, Y. et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 26, 17–26.e6 (2020).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Clevers, H. & Tuveson, D. A. Organoid models for cancer research. Annu. Rev. Cancer Biol. 3, 223–234 (2019).
Jacob, F. et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 180, 188–204.e22 (2020).
Qian, X. et al. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 13, 565–580 (2018).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Han, S. H. et al. Long-term culture-induced phenotypic difference and efficient cryopreservation of small intestinal organoids by treatment timing of Rho kinase inhibitor. World J. Gastroenterol. 23, 964–975 (2017).
Li, X., Meng, G., Krawetz, R., Liu, S. & Rancourt, D. E. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 17, 1079–1085 (2008).
Shi, J. & Wei, L. Rho kinase in the regulation of cell death and survival. Arch. Immunol. Ther. Exp. (Warsz) 55, 61–75 (2007).
Tilson, S. G. et al. ROCK inhibition facilitates in vitro expansion of glioblastoma stem-like cells. PloS One 10, e0132823 (2015).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Newick, K., O’Brien, S., Moon, E. & Albelda, S. M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 68, 139–152 (2017).
Goff, S. L. et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J. Immunother. 42, 126–135 (2019).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Johnson, L. A. et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci. Transl. Med. 7, 275ra222 (2015).
Schnalzger, T. E. et al. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 38, e100928 (2019).
Neftel, C. et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849.e21 (2019).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Kong, W. J. et al. CellTagging: combinatorial indexing to simultaneously map lineage and identity at single-cell resolution. Nat. Protoc. 15, 750–772 (2020).
Cattaneo, C. M. et al. Tumor organoid-T-cell coculture systems. Nat. Protoc. 15, 15–39 (2020).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Ledur, P. F., Onzi, G. R., Zong, H. & Lenz, G. Culture conditions defining glioblastoma cells behavior: what is the impact for novel discoveries? Oncotarget 8, 69185–69197 (2017).
Lee, J. et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9, 391–403 (2006).
Patrizii, M., Bartucci, M., Pine, S. R. & Sabaawy, H. E. Utility of glioblastoma patient-derived orthotopic xenografts in drug discovery and personalized therapy. Front. Oncol. 8, 23 (2018).
Qian, X., Nguyen, H. N., Jacob, F., Song, H. & Ming, G. L. Using brain organoids to understand Zika virus-induced microcephaly. Development 144, 952–957 (2017).
Bian, S. et al. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 15, 631–639 (2018).
Ogawa, J., Pao, G. M., Shokhirev, M. N. & Verma, I. M. Glioblastoma model using human cerebral organoids. Cell Rep. 23, 1220–1229 (2018).
Hubert, C. G. et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 76, 2465–2477 (2016).
Yi, H. G. et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 3, 509–519 (2019).
Hovinga, K. E. et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells 28, 1019–1029 (2010).
Shimizu, F., Hovinga, K. E., Metzner, M., Soulet, D. & Tabar, V. Organotypic explant culture of glioblastoma multiforme and subsequent single-cell suspension. Curr. Protoc. Stem Cell Biol. Ch. 3, Unit 3.5 (2011).
Merz, F. et al. Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments. Neuro Oncol. 15, 670–681 (2013).
Parker, J. J., Lizarraga, M., Waziri, A. & Foshay, K. M. A human glioblastoma organotypic slice culture model for study of tumor cell migration and patient-specific effects of anti-invasive drugs. J. Vis. Exp. e53557 (2017).
Herrera-Perez, M., Voytik-Harbin, S. L. & Rickus, J. L. Extracellular matrix properties regulate the migratory response of glioblastoma stem cells in three-dimensional culture. Tissue Eng Part A 21, 2572–2582 (2015).
Huang, L. E. Friend or foe—IDH1 mutations in glioma 10 years on. Carcinogenesis 40, 1299–1307 (2019).
Abud, E. M. et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94, 278–293.e9 (2017).
Cakir, B. et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175 (2019).
Lin, Y. T. et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98, 1141–1154.e7 (2018).
Pham, M. T. et al. Generation of human vascularized brain organoids. Neuroreport 29, 588–593 (2018).
Porter, D. L., Levinu, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Jonkman, J., Brown, C. M., Wright, G. D., Anderson, K. I. & North, A. J. Tutorial: guidance for quantitative confocal microscopy. Nat. Protoc. 15, 1585–1611 (2020).
Karsy, M., Albert, L., Tobias, M. E., Murali, R. & Jhanwar-Uniyal, M. All-trans retinoic acid modulates cancer stem cells of glioblastoma multiforme in an MAPK-dependent manner. Anticancer Res. 30, 4915–4920 (2010).
Hobro, A. J. & Smith, N. I. An evaluation of fixation methods: spatial and compositional cellular changes observed by Raman imaging. Vib. Spectrosc. 91, 31–45 (2017).
Drexler, H. G. & Uphoff, C. C. Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology 39, 75–90 (2002).
Bhaduri, A. et al. Outer radial glia-like cancer stem cells contribute to heterogeneity of glioblastoma. Cell Stem Cell 26, 48–63.e6 (2020).
Wang, R. et al. Adult human glioblastomas harbor radial glia-like cells. Stem Cell Reports 14, 338–350 (2020).
Brennan, C. W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Acknowledgements
We thank all patients who generously donated tissue. We thank members of the Ming and Song laboratories for comments and suggestions, B. Temsamrit and E. LaNoce for technical support, J. Schnoll for lab coordination, and D. O’Rourke, M. Nasrallah and others in the Departments of Neurosurgery and Pathology at the Hospital of the University of Pennsylvania for assistance with tissue acquisition. This work was supported by the Glioblastoma Translational Center of Excellence at the Abramson Cancer Center at the University of Pennsylvania, grants from the National Institutes of Health (R37NS047344 and R35NS116843 to H.S., R35NS097370 and U19AI131130 to G.-l.M.), the Sheldon G. Adelson Medical Research Foundation (to G.-l.M.) and the Pennsylvania Department of Health (to G.-l.M.).
Author information
Authors and Affiliations
Contributions
F.J. developed the procedures to generate, culture and biobank GBOs and to co-culture GBOs with CAR T cells and produced the sample data. F.J., G.-l.M. and H.S. conceived the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Lisa Ebert, Henner Farin, Guillermo Gomez, Yanhong Shi and Ali G Turhan for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Jacob, F. et al. Cell 180, 188–204.e122 (2020): https://doi.org/10.1016/j.cell.2019.11.036
Key data used in this protocol
Jacob, F. et al. Cell 180, 188–204.e122 (2020): https://doi.org/10.1016/j.cell.2019.11.036
Supplementary information
Supplementary Information
Supplementary Methods
Supplementary Video 1
Video showing procedures for processing patient glioblastoma tissue for organoid culture
Rights and permissions
About this article
Cite this article
Jacob, F., Ming, Gl. & Song, H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat Protoc 15, 4000–4033 (2020). https://doi.org/10.1038/s41596-020-0402-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0402-9
This article is cited by
-
Patient-derived organoids in human cancer: a platform for fundamental research and precision medicine
Molecular Biomedicine (2024)
-
Establishment of tumor microenvironment-preserving organoid model from patients with intracranial meningioma
Cancer Cell International (2024)
-
Application status and optimization suggestions of tumor organoids and CAR-T cell co-culture models
Cancer Cell International (2024)
-
Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies
Nature Reviews Immunology (2024)
-
BEHAV3D: a 3D live imaging platform for comprehensive analysis of engineered T cell behavior and tumor response
Nature Protocols (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.