Abstract
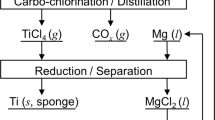
Titanium (Ti) is produced by a multi-step process from Ti ore composed of Ti oxide including chlorination process. However, the smelting cost is a major factor that leads to an increase in the cost of Ti. To lower the cost of Ti, the development of a new technique for the direct production of metallic Ti from an oxide feed material is highly desired. However, owing to the high affinity between Ti and oxygen (O) and the high solubility of O in Ti, it is extremely difficult to produce metallic Ti with low O concentration which meets the level required by the industry. In this study, we developed a new method for producing Ti with a low O concentration directly from TiO2 by using Mg as a reductant, assisted by a rare earth oxychloride formation reaction. The production of Ti with a low O concentration was demonstrated using a magnesiothermic reduction of TiO2 in MgCl2–LaCl3 molten salt via a reduction reaction involving LaOCl formation: (TiO2 (s) + 2 Mg (l) + 2 LaCl3 (l) = Ti (s) + 2 MgCl2 (l) + 2 LaOCl (s)). An electrochemical method that produces metallic Ti from TiO2 with no consumption of rare earth elements and Mg was designed based on the experiment results. This process is expected to replace the current smelting processes of Ti as well as numerous other rare metals produced from raw oxide materials and contribute significantly to a decrease in the price of rare metals.
Graphical Abstract











Similar content being viewed by others
References
Okabe TH (2019) Metallothermic reduction of TiO2. In: Fang ZZ, Froes FH, Zhang Y (eds) Extractive metallurgy of titanium, 1st edn, pp 131–164
Okabe TH, Takeda O (2019) Fundamentals of thermochemical reduction of TiCl4. In: Fang ZZ, Froes FH, Zhang Y (eds) Extractive metallurgy of titanium, 1st edn, pp 65–96
Takeda O, Ouchi T, Okabe TH (2020) Recent progress in titanium extraction and recycling. Metall Mater Trans B 51:1315–1328
Okabe TH, Zheng C, Taninouchi Y (2018) Thermodynamic considerations of direct oxygen removal from titanium by utilizing the deoxidation capability of rare earth metals. Metall Mater Trans B 49:1056–1066
Zheng C, Ouchi T, Iizuka A, Taninouchi Y, Okabe TH (2019) Deoxidation of titanium using Mg as deoxidant in MgCl2-YCl3 flux. Metall Mater Trans B 50:622–631
Perkin FM, Pratt L (1907) Reducing action of metallic calcium and calcium hydride upon metallic oxides, sulphides, and halogen salts. Trans Faraday Soc 3:179–186
Hasegawa M (1950) Production of crude titanium. J Jpn Inst Met A 14:23–26 (in Japanese)
Sibert ME, Steinberg MA (1956) Electrolytic titanium. J Met 8:1162–1168
Henrie TA (1968) Extractive metallurgy of titanium. High Temp Refract Metals 34:139–154
Oki T, Inoue H (1967) Reduction of titanium dioxide by calcium in hot cathode spot. Mem Fac Eng Nagoya Univ 19:164–166
Hayes FH, Bomberger HB, Froes FH, Kaufman L, Burte HM (1984) Advances in titanium extraction metallurgy. JOM 36:70–76
Ono K, Miyazaki S (1985) Study on the limit of deoxidation of titanium and the reduction of titanium dioxide by saturated calcium vapors. J Jpn Inst Met 49:871–875
Ono K, Ogawa M, Okabe TH, Suzuki RO (1990) Production of titanium powders by the calciothermic reduction of TiO2. Tetsu-to-Hagane 76:568–575
Okabe TH (1993) A fundamental study on refining of titanium and its aluminides. PhD thesis, Kyoto University, pp 1–209
Okabe TH, Oda T, Mitsuda Y (2004) Titanium powder production by preform reduction process (PRP). J Alloys Compd 364:156–163
Okabe TH, Waseda Y (1997) Producing titanium through an electronically mediated reaction. JOM 49:28–32
Abiko T, Park I, Okabe TH (2003) Reduction of titanium oxide in molten salt medium. 10th World Conference on Titanium, Humburg
Park I, Abiko T, Okabe TH (2005) Production of titanium powder directly from TiO2 in CaCl2 through an electronically mediated reaction (EMR). J Phys Chem Solids 66:410–413
Chen GZ, Fray DJ, Farthing TW (2000) Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 407:361–364
Fray DJ (2001) Emerging molten salt technologies for metals production. JOM 53:26–31
Chen GZ, Fray DJ, Farthing TW (2004) Removal of oxygen from metal oxides and solid solutions by electrolysis in a fused salt. United States Patent 20040159559A1
Ono K, Suzuki RO (2002) A new concept for producing Ti sponge: calciothermic reduction. JOM 54:59–61
Suzuki RO, Inoue S (2003) Calciothermic reduction of titanium oxide in molten CaCl2. Metall Mater Trans B 34:277–285
Suzuki RO, Ono K, Teranuma K (2003) Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall Mater Trans B 34:287–295
Suzuki RO, Fukui S (2004) Reduction of TiO2 in molten CaCl2 by Ca deposited during CaO electrolysis. Mater Trans 45:1665–1671
Suzuki RO (2005) Calciothermic reduction of TiO2 and in-situ electrolysis of CaO in molten CaCl2. J Phys Chem Solids 66:461–465
Fang ZZ, Middlemas S, Guo J, Fan P (2013) A new, energy-efficient chemical pathway for extracting Ti metal from Ti minerals. J Am Chem Soc 135:18248–18251
Zhang Y, Fang ZZ, Sun P, Zhang T, Xia Y, Zhou C, Huang Z (2016) Thermodynamic destabilization of Ti-O solid solution by H2 and deoxygenation of Ti using Mg. J Am Chem Soc 138:6916–6919
Zhang Y, Fang ZZ, Xia Y, Huang Z, Lefler H, Zhang T, Sun P, Free ML, Guo J (2016) A novel chemical pathway for energy efficient production of Ti metal from upgraded titanium slag. Chem Eng J 286:517–527
Zhang Y, Fang ZZ, Xia Y, Sun P, Devener BV, Free M, Lefler H, Zheng S (2017) Hydrogen assisted magnesiothermic reduction of TiO2. Chem Eng J 308:299–310
Zhang Y, Fang ZZ, Sun P, Xia Y, Free M, Huang Z, Lefler H, Zhang T, Guo J (2017) Kinetically enhanced metallothermic redox of TiO2 by Mg in molten salt. Chem Eng J 327:169–182
Xia Y, Fang ZZ, Zhang Y, Leer H, Zhang T, Sun P, Huang Z (2017) Hydrogen assisted magnesiothermic reduction (HAMR) of commercial TiO2 to produce titanium powder with controlled morphology and particle size. Mater Trans 58:355–360
Lefler H, Fang ZZ, Zhang Y, Sun P, Xia Y (2018) Mechanisms of hydrogen-assisted magnesiothermic reduction of TiO2. Metall Mater Trans B 49:2998–3006
Li Q, Zhu X, Zhang Y, Fang ZZ, Zheng S, Sun P, Xia Y, Li P, Zhang Y, Zou X (2019) An investigation of the reduction of TiO2 by Mg in H2 atmosphere. Chem Eng Sci 195:484–493
Nersisyan HH, Lee JH, Won CW (2003) Combustion of TiO2–Mg and TiO2–Mg–C systems in the presence of NaCl to synthesize nanocrystalline Ti and TiC powders. Mater Res Bull 38:1135–1146
Eshed M, Irzh A, Gedanken A (2009) Reduction of titanium dioxide to metallic titanium conducted under the autogenic pressure of the reactants. Inorg Chem 48:7066–7069
Bolivar R, Friedrich B (2019) Magnesiothermic reduction from titanium dioxide to produce titanium powder. J Sustain Metall 5:219–229
Won CW, Nersisyan HH, Won HI (2010) Titanium powder prepared by a rapid exothermic reaction. Chem Eng J 157:270–275
Ouchi K, Kobayashi Y, Endo R, Susa M (2013) Promotion of solid TiO2 reduction by molten magnesium from the perspective of reaction mechanisms. Tetsu-to-Hagane 99:433–438 (in Japanese)
Nersisyan HH, Won HI, Won CW, Jo A, Kim JH (2014) Direct magnesiothermic reduction of titanium dioxide to titanium powder through combustion synthesis. Chem Eng J 235:67–74
Okabe TH, Taninouchi Y, Zheng C (2018) Thermodynamic analysis of deoxidation of titanium through the formation of rare-earth oxyfluorides. Metall Mater Trans B 49:3107–3117
Zheng C, Ouchi T, Kong L, Taninouchi Y, Okabe TH (2019) Electrochemical deoxidation of titanium in Molten MgCl2-YCl3. Metall Mater Trans B 50:1652–1661
Kong L, Ouchi T, Okabe TH (2019) Direct deoxidation of Ti by Mg in MgCl2-HoCl3 flux. Mater Trans (JIM) 60:2059–2068
Kong L, Ouchi T, Zheng C, Okabe TH (2019) Electrochemical deoxidation of titanium scrap in MgCl2-HoCl3 system. J Electrochem Soc 166:E429–E437
Iizuka A, Ouchi T, Okabe TH (2020) Ultimate deoxidation method of titanium utilizing Y/YOCl/YCl3 equilibrium. Metall Mater Trans B 51:433–442
Iizuka A, Ouchi T, Okabe TH (2020) Development of a new titanium powder sintering process with deoxidation reaction using yttrium metal. Mater Trans 61:758–765
Tanaka T, Ouchi T, Okabe TH (2020) Lanthanothermic reduction of TiO2. Metall Mater Trans B 51:1485–1494. https://doi.org/10.1007/s11663-020-01860-6
Tanaka T, Ouchi T, Okabe TH (2020) Yttriothermic reduction of TiO2 in molten salts. Mater Trans 61:1967–1973
Kong L, Ouchi T, Okabe TH (2020) Deoxidation of Ti using Ho in HoCl3 flux and determination of thermodynamic data of HoOCl. J Alloy Compd. https://doi.org/10.1016/j.jallcom.2020.156047
Arum-shuppan (2019) The rare metal news. no. 2838, 5
Gupta CK, Krishnamurthy N (2013) Oxide reduction processes in the preparation of rare-earth metals. Mining Metall Explor 30:38–44
Muthmann W, Weiss L (1904) Untersuchungen über die metalle der cergruppe. Justus Liebigs Ann Chem 331:1–46
Muthmann W, Weiss L (1907) Untersuchungen über metallisches vanadin, niob, tantal. Justus Liebigs Ann Chem 351:59–99
Vogel R (1917) About the influence of the titanium on the perlite creation in carbon steel. Ferrum 14:177–197
Okabe TH, Suzuki RO, Oishi T, Ono K (1991) Thermodynamic properties of dilute titanium-oxygen solid solution in beta phase. Mater Trans (JIM) 32:485–488
Barin I (1995) Thermochemical data of pure substance, 3rd edn. Wiley-VCH, Weinheim
Chase MW (1998) NIST-JANAF Thermochemical Tables, 4th edn., American Institute of Physics
Permyakov PG, Korshunov BG, Krokhin VA (1975) Solubility of rare earth and yttrium oxochlorides in molten salts: MCl2 (M: Mg, Ca, Sr, Ba). Zhurnal Neorganicheskoj Khimii 20:2184–2187
Drobot DV, Korshunov BG, Durinina LV (1965) Equilibrium of reaction of lanthanum and praseodymium chlorides with oxygen. Izv. Akad. Nauk SSSR. Neorg Mater 1:2189–2196
Okabe TH, Suzuki RO, Oishi T, Ono K (1991) Production of extra low oxygen titanium by calcium-halide flux deoxidation. Tetsu-to-Hagane 77:93–99 (in Japanese)
Okabe TH, Oishi T, Ono K (1992) Preparation and characterization of extra-low-oxygen titanium. J Alloys Compd 184:43–56
Okabe TH, Nakamura M, Ueki T, Oishi T, Ono K (1992) Preparation of extra-low-oxygen titanium by the calcium-halide flux deoxidation process. Materia Japan 31:315–317 (in Japanese)
Acknowledgements
The authors are grateful to Mr. Akihiro Iizuka and Dr. Lingxin Kong at The University of Tokyo for their effective comments. This work was financially supported by the Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for Scientific Research (S) (KAKENHI Grant No. 26220910 and 19H05623).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Hongmin Zhu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanaka, T., Ouchi, T. & Okabe, T.H. Magnesiothermic Reduction of TiO2 Assisted by LaCl3. J. Sustain. Metall. 6, 667–679 (2020). https://doi.org/10.1007/s40831-020-00296-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00296-1