Abstract
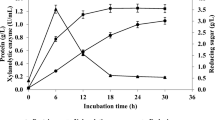
This study aims to establish a bacterial consortium to deconstruct the coir, areca, banana, and cotton fibers recalcitrant structure. Hence, lignocellulase secreting bacterial strains were isolated from cow rumen, dung garbage, and vermicompost samples. After selecting desired strains, a mixed-batch bacterial consortium (MBBC) was made by mixing of cellulase batch (Bacillus sp. HSTU-2, Bacillus sp. HSTU-3, Citrobacter sp. HSTU-AAJ4), pectinase batch (Acinetobacter sp. HSTU-6, Bacillus sp. HSTU-7, Enterobacter sp. HSTU-AAH8), and amylase batch (Bacillus sp. HSTU-9, and Bacillus sp. HSTU-10) strains. Separately, each batch and MBBC strains were largely grown in the culture medium enriched with coir, areca, banana, and cotton fibers; as a consequence, the hardy lignocellulosic fibers were degraded. FTIR study indicated that the peak intensities for lignin was diminished, but sharpened for cellulose in the MBBC than that of the single batch pretreatment. The MBBC pretreatment could remove 64–73% lignin from banana, areca, and coir fibers, resulting in increasing cellulose amounts. The crystallinity index was observed 31.5%, 21.71%, 35%, and 29.26% for the untreated and 13.69%, 18.27%, 17.72%, and 25.20% for the MBBC pretreated cotton, areca, coir, and banana fibers, respectively. The MBBC pretreated banana, areca, coir, and cotton fibers could generate reducing sugars that scaled up to 5.3-, 3.9-, 3.68-, and 2.68-fold greater than the untreated samples. Hence, it is radically feasible to produce bioethanol precursors from the 4-days long MBBC pretreated banana, areca, and coir lignocelluloses. This research revealed the shortest lignocellulose pretreatment duration with a bacterial consortium holding minimal community members.
Graphic Abstract







Similar content being viewed by others

References
Swaraz, A.M., Satter, M.A., Rahman, M.M., Asad, M.A., Khan, I., Amin, M.Z.: Bioethanol production potential in Bangladesh from wild date palm (Phoenix sylvestris Roxb.): An experimental proof. Ind. Crops Prod. 139(111507), 1–9 (2019)
Pereira, A.L.S., Nascimento, D.M.D., Souza, M.D.S.M., Cassales, A.R., Morais, J.P.S., Paula, R.C.M.D., Rosa, M.D.F., Feitosa, J.P.A.: Banana (Musa sp. Cv. Pacovan) Pseudostem fibers are composed of varying lignocellulosic composition throughout the diameter. BioResources 9(4), 7749–7763 (2014)
Kongkaew, P.: Mechanical properties of banana and coconut fibers reinforced epoxy polymer matrix composites. Proceedings of Academics World 17th International Conference, Tokyo, Japan, 2016, https://pdfs.semanticscholar.org/10ca/dd3d93cb7cfbb17185781fd9d8851867336d.pdf
Brown, R.C., Brown, T.R.: (2003). Biorenewable Resources: Engineering New Products from Agriculture. 2nd Edition, Ames, USA: Iowa State Press.
Malherbe, S., Cloete, T.E.: Lignocellulose biodegradation: Fundamentals and applications. Rev. Environ. Sci. 1(2), 105–114 (2002)
EI-Naggar, N.E.A., Deraz, S., Khalil, A.: Bioethanol production from lignocellulosic feedstocks based on enzymatic hydrolysis: Current status and recent developments. Biotechnology (2014). https://doi.org/10.3923/biotech.2014.1.21
Puentes-Téllez, P.E., Salles, J.F.: Construction of effective minimal active microbial consortia for lignocellulose degradation. Microb. Ecol. 76, 419–429 (2018)
Haque, M.A., Cho, K.M., Barman, D.N., Kim, M.K., Yun, H.D.: A potential cellulose microfibril swelling enzyme isolated from Bacillus sp. AY8 enhances cellulose hydrolysis. Process Biochem. 50(5), 807–815 (2015)
Barman, D.N., Haque, M.A., Hossain, M.M., Paul, S.K., Yun, H.D.: Deconstruction of pine wood (Pinus sylvestris) recalcitrant structure using alkali treatment for enhancing enzymatic saccharification evaluated by Congo red. Waste Biomass Valori. 11, 755–1764 (2018)
Zhu, J.Y., Pan, X.J., Wang, G.S., Gleisner, R.: Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 100(8), 2411–2418 (2009)
Chang, V.S., Burr, B., Holtzapple, M.T.: Lime pretreatment of switchgrass. Appl. Biochem. Biotechnol. 63–65, 3–19 (1997)
Taherzadeh, M.J., Karimi, K.: Acid-based hydrolysis processes for ethanol from lignocellulosic materials A review. BioResources 2(3), 472–499 (2007)
De Souza, W.R., Chandel, A.K., Silva, S.S.D.: Microbial degradation of lignocellulosic biomass - techniques, applications and commercialization. Chapter 9. Janeza Trdine 9, 51000 Rijeka, Croatia: InTech. (2013)
Rabinovich, M.L., Melnick, M.S., Bolobova, A.V.: The structure and mechanism of action of cellulolytic enzymes. Biochemistry (Moscow) 67(8), 850–871 (2002)
Kuhad, R.C., Gupta, R., Singh, A.: Microbial cellulases and their industrial applications. Enzyme Res. (2011). https://doi.org/10.4061/2011/280696
Okeke, B.C., Lu, J.: Characterization of a defined cellulolytic and xylanolytic bacterial consortium for bioprocessing of cellulose and hemicelluloses. Appl. Biochem. Biotechnol. 163(7), 869–881 (2011)
Liang, J., Peng, X., Yin, D., Li, B., Wang, D., Lin, Y.: Screening of a microbial consortium for highly simultaneous degradation of lignocellulose and chlorophenols. Bioresour. Technol. 190, 381–387 (2015)
Sharma, H.K., Xu, C.C., Qin, W.: Co-culturing of novel Bacillus species isolated from municipal sludge and gut of red wiggler worm for improving CMCase activity. Waste Biomass Valori. 11, 2047–2058 (2020)
Oumer, O.J., Abate, D.: Screening and molecular identification of pectinase producing microbes from coffee pulp. BioMed Res. Int. (2018). https://doi.org/10.1155/2018/2961767
Bergey, D.H., Holt, J.G.: Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins. Chikago, Baltimore (1993)
Sambrook, J.: Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (2001)
Kumar, S., Stecher, G., Li, M., Knyaz, C., Tamura, K.: MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35(6), 1547–1549 (2018)
Lin, L., Yan, R., Liu, Y., Jiang, W.: In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: cellulose, hemicellulose and lignin. Bioresour. Technol. 101, 8217–8223 (2010)
Blasi, C.D., Signorelli, G., Russo, C.D., Rea, G.: Product distribution from pyrolysis of wood and agricultural residues. Ind. Eng. Chem. Res. 38, 2216–2224 (1999)
Segal, L., Creely, J.J., Martin, A.E., Conrad, C.M.: An emperical method for estimating the degree of crystallinity of native cellulose using X-ray diffractometer. Text. Res. J. 29(10), 786–794 (1959)
Haque, M.A., Barman, D.N., Kang, T.H., Kim, M.K., Kim, J., Kim, H., Yun, H.D.: Effect of dilute alkali pretreatment on structural features and enhanced enzymatic hydrolysis of Miscanthus sinensis at boiling temperature with low residence time. Biosyst. Eng. 114(3), 294–305 (2013)
Ferrer, M., Golyshina, O.V., Chernikova, T.N., Khachane, A.N., Reyes-Duarte, D., Santos, V.A., Strompl, C., Elborough, K., Jarvis, G., Neef, A., Yakimov, M.M., Timmis, K.N., Golyshin, P.N.: Novel hydrolase diversity retrieved from a metagenome library of bovine rumen microflora. Environ. Microbiol. 7, 1996–2010 (2005)
Rosnow, J.J., Anderson, L.N., Nair, R.N., Baker, E.S., Wright, A.T.: Profiling microbial lignocellulose degradation and utilization by emergent omics technologies. Crit. Rev. Biotechnol. 37(5), 626–640 (2017)
Salvachúa, D., Karp, E.M., Nimlos, C.T., Vardon, D.R., Beckham, G.T.: Towards lignin consolidated bioprocessing: Simultaneous lignin deploymerization and product generation by bacteria. Green Chem. 17(11), 4951–4967 (2015)
Kumar, M., Verma, S., Gazara, R.K., Kumar, M., Pandey, A., Verma, P.K., Thakur, I.S.: Genomic and proteomic analysis of lignin degrading and polyhydroxyalkanoate accumulating proteobacterium Pandoraea sp. ISTKB. Biotechnol Biofuels (2018). https://doi.org/10.1186/s13068-018-1148-2
Rahmanpour, R., Rea, D., Jamshidi, S., Fülöp, V., Bugg, T.D.: Structure of Thermobifda fusca DyP- type peroxidase and activity towards kraft lignin and lignin model compounds. Arch. Biochem. Biophys. 594, 54–60 (2016)
Li, Y., Lei, L., Zheng, L., Xiao, X., Tang, H., Luo, C.: Genome sequencing of gut symbiotic Bacillus velezensis LC1 for bioethanol production from bamboo shoots. Biotechnol. Biofuels (2020). https://doi.org/10.1186/s13068-020-1671-9
Zhang, Z.Y., Raza, M.F., Zheng, Z., Zhang, X., Dong, X., Zhang, H.: Complete genome sequence of Bacillus velezensis ZY-1–1 reveals the genetic basis for its hemicellulosic/cellulosic substrate-inducible xylanase and cellulase activities. Biotech (2018). https://doi.org/10.1007/s13205-018-1490-x
Okolie, P.N., Ugochukwu, E.N.: Changes in activities of cell wall degrading enzymes during fermentation of cassava (Manihot esculenta Crantz) with citrobacter freundii. J. Sci. Food Agr. (1988). https://doi.org/10.1002/jsfa.2740440107
Zheng, L., Du, B., Xu, W.: Screening and identification of Acinetobacter junii for Apocynum vernetum L fiber enzymatic retting. J. Text. I. 102(8), 675–680 (2011)
Hosseini-Abari, A., Emtiazi, G., Jazini, M., Kim, J., Kim, B.G.: LC/MS detection of oligogalacturonic acids obtained from tragacanth degradation by pectinase producing bacteria. J. Basic. Microbiol. 59(3), 249–255 (2019)
Chang, Y.C., Choi, D., Takamizawa, K., Kikuchi, S.: Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Bioresour. Technol. 152, 429–436 (2014)
Barman, D.N., Haque, M.A., Kang, T.H., Kim, G.H., Kim, T.Y., Kim, M.K., Yun, H.D.: Effect of mild alkali pretreatment on structural changes of reed (Phragmites communis Trinius) straw. Environ. Technol. 35(2), 232–241 (2014)
Zhang, Y.H.P., Ding, S.Y., Mielenz, J.R., Cui, J.B., Elander, R.T., Laser, M., Himmel, M.E., McMillan, J.R., Lynd, L.R.: Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 97, 214–223 (2007)
Shi, J., Chinn, M.S., Sharma-shivappa, R.R.: Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour. Technol. 99, 6556–6564 (2008)
Du, W., Yu, H., Song, L., Zhang, J., Weng, C., Ma, F., Zhang, X.: The promoting effect of byproducts from Irpex lacteus on subsequent enzymatic hydrolysis of bio-pretreated cornstalks. Biotechnol Biofuels (2011). https://doi.org/10.1186/1754-6834-4-37
Shirkavand, E., Baroutian, S., Gapes, D.J., Young, B.R.: Pretreatment of radiate pine using two white rot fungal strains Stereum hirsutum and Trametes versicolor. Energ. Convers. Manage. 142, 13–19 (2017)
Liu, X., Hiligsmann, S., Gourdon, R., Bayard, R.: Anaerobic digestion of lignocellulosic biomasses pretreated with Ceriporiopsis subvermispora. J. Environ. Manag. 193, 154–162 (2017)
Odier, E., Janin, G., Monties, B.: Poplar lignin decomposition by gram-negative aerobic bacteria. Appl. Environ. Microbiol. 41(2), 337–341 (1981)
Dionisi, D., Anderson, J.A., Aulenta, F., McCue, A., Paton, G.: The potential of microbial processes for lignocellulosic biomass conversion to ethanol: a review. J. Chem. Technol. Biotechnol. 90, 366–383 (2015)
Zimmermann, W., Broda, P.: Utilization of lignocellulose from barley straw by Actinomycetes. Appl. Microbiol. Biotechnol. 30(1), 103–109 (1989)
Giroux, H., Vidal, P., Bouchard, J., Lamy, F.: Degradation of Kraft indulin lignin by Streptomyces viridosporus and Streptomyces badius. Appl. Environ. Microbiol. 54(12), 3064–3070 (1988)
Si, M., Liu, D., Liu, M., Yan, X., Gao, C., Chai, L., Shi, Y.: Complementary effect of combined bacterial- chemical pretreatment to promote enzymatic digestibility of lignocellulose biomass. Biores. Technol. 272, 275–280 (2019)
Guo, H., Lin, C., Wang, S., Jiang, D., Zheng, B., Liu, Y., Qin, W.: Characterization of a novel laccase-producing Bacillus sp A4 and its application in Miscanthus degradation. BioRes. 12(3), 4776–4794 (2017)
Brossi, M.J.L., Jiménez, D.J., Cortes-Tolalpa, L., van Elsas, J.D.: Soil-derived microbial consortia enriched with different plant biomass reveal distinct players acting in lignocellulose degradation. Microb. Ecol. 71(3), 616–627 (2016)
Narayanan, P.M., Murugan, S.: Production, purification and application of bacterial laccase a review. Biotech (2014). https://doi.org/10.3923/biotech.2014.196.205
Potprommanee, L., Wang, X.Q., Han, Y.J., Nyobe, D., Peng, Y.P., Huang, Q., Liu, J.Y., Liao, Y.L., Chang, K.L.: Characterization of a thermophilic cellulase from Geobacillus sp HTA426, an efficient cellulase-producer on alkali pretreated of lignocellulosic biomass. PLoS ONE (2017). https://doi.org/10.1371/journal.pone.0175004
Reddy, M.P.C., Saritha, K.V.: Effects of the culture media optimization on pectinase production by Enterobacter sp PSTB-1. 3 Biotech (2016). https://doi.org/10.1007/s13205-016-0502-y
Lokapirnasari, W.P., Nazar, D.S., Nurhajati, T., Supranianondo, K., Yulianto, A.B.: Production and assay of cellulolytic enzyme activity of Enterobacter cloacae WPL 214 isolated from bovine rumen fluid waste of Surabaya abbatoir. Indonesia. Vet. World 8(3), 367–371 (2015)
Cortes-Tolalpa, L., Wang, Y., Salles, J.F., van Elsas, J.D.: Comparative genome analysis of the lignocellulose degrading bacteria Citrobacter freundii so4 and Sphingobacterium multivorum w15 Microbiol. Front (2020). https://doi.org/10.3389/fmicb.2020.00248
Vasudevan, N., Mahadevan, A.: Degradation of lignin and lignin derivatives by Acinetobacter sp. J. Appl. Microbiol. 70(2), 169–176 (2008)
Xu, F., Yu, J., Tesso, T., Dowell, F., Wang, D.: Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energ. 104, 801–809 (2013)
Abraham, E., Deepab, B., Pothen, L.A., Cintil, J., Thomas, S., John, M.J., Anandjiwala, R., Narine, S.S.: Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 92, 1477–1483 (2013)
Sun, X.F., Xu, F., Sun, R.C., Fowler, P., Baird, M.S.: Characteristics of degraded cellulose obtained from steam exploded wheat straw. Carbohydr. Res. 340, 97–106 (2005)
Ruiz, H.A., Cerqueira, M.A., Silva, H.D., Rodríguez-Jasso, R.M., Vicente, A.A., Teixeira, J.A.: Biorefinery valorization of auto hydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohydr. Polym. 92, 2154–2162 (2013)
Ingale, S., Joshi, S.J., Gupte, A.: Production of bioethanol using agricultural waste: Banana pseudostem. Braz. J. Microbiol. 45(3), 885–892 (2014)
Jayana, N.K.K., Basaiah, T., Krishnappa, M.: Acid and enzyme hydrolysis to convert pretreated areca nut (areca catechu l.) husk into glucose for bioethanol production by yeasts and Zymomonas mobilis NCIM 2915. Research in Biotechnology 6(6), 17–30 (2015)
Gundupali, M.P., Debraj Bhattacharjja, D.: Sequential acid hydrolysis and enzymatic saccharification of coconut coir for recovering reducing sugar: Process evaluation and optimization. Biores. Technol. Rep. 6, 70–80 (2019)
Acknowledgements
The research was supported by The World Academy of Sciences (TWAS), Trieste, Italy, and as a research grant (Individual) winner. Dr. Md. Azizul Haque would like to express gratitude to TWAS (Research Grants: 17-475 RG/BIO/AS_I, January, 2018 -June, 2020). The authors would like to acknowledge the Ministry of Science and Technology, Government of Bangladesh (MOST-2017) for the assistance.
Funding
The research was supported by The World Academy of Sciences (TWAS), Trieste, Italy, and as a research grant (Individual) winner Dr. Md. Azizul Haque would like to express gratitude to TWAS (Research Grants: 17–475 RG/BIO/AS_I, January, 2018 -June, 2020). The authors would like to acknowledge the Ministry of Science and Technology, Government of Bangladesh (MOST-2017) for the initial funding assistance.
Author information
Authors and Affiliations
Contributions
Dr. Md. Azizul Haque have conceived, designed, supervised the experiments, and analyzed the data as well as written the manuscript. Md. Ashikujjaman Ashik, Most. Sarmin Akter, Abubakar Halilu performed experiments and written their own thesis for graduation. Shefali Aktar, Dr. Mst. Nur-E- Nazmun Nahar, and Shukla Rani Das has superivesed and cosupervised the graduate students and comments on the data during experiments. Dr. Md. Atiqul Haque and Shefali Aktar was involved in the fund hunting with Dr. Md. Azizul Haque. Dr. Md. Reazul Islam, Dr. Md. Atiqul Haque, and Muhammad Rubayat Bin Shahadat carefully proofread the manuscript with and Dr. Md. Azizul Haque. Md. Abdullah-Al-Mamun, Keshob Chandra Das, Irfan Ahmed, Md. Ashikujjaman, and Dr. Md. Azizul Haque was involved in DNA sequencing and Analysis. Dr. Md. Azizul Haque, Md. Serajum Manir, and Md. Khairul Islam performed the FT-IR and X-ray Diffraction analyses of the samples.
Corresponding author
Ethics declarations
Conflicts of interest
The manuscript is not under consideration by another journal as the same time as ‘Waste and Biomass Valorization’ Journal. All authors have approved the manuscript for the submission to ‘Waste and Biomass Valorization’ Journal.
Ethical Approval
This research does not contain any human or animal experiments. Therefore, ethical approval was not required.
Consent to Publication
The authorities and authors agreed to submit the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haque, M.A., Ashik, M.A., Aktar, S. et al. Rapid Deconstruction of Cotton, Coir, Areca, and Banana Fibers Recalcitrant Structure Using a Bacterial Consortium with Enhanced Saccharification. Waste Biomass Valor 12, 4001–4018 (2021). https://doi.org/10.1007/s12649-020-01294-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01294-w