Abstract
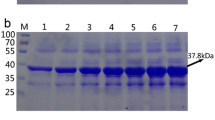
Pichia pastoris expression system was introduced with post-translation process similar to higher eukaryotes. Preliminary studies were performed toward process intensification and magnetic immobilization of this system. In this experiment, effects of magnetic immobilization on the structure of recombinant protein were evaluated. P. pastoris cell which express human serum albumin (HSA) was used as a model. The cells were immobilized with various concentrations of APTES coated magnetite nanoparticles. HSA production was done over 5 days induction and structure of the product was analyzed by UV–vis, fluorescence, and ATR-FTIR spectroscopy. Second derivative deconvolution method was used to analyze the secondary structure of HSA. P. pastoris cell that were immobilized with 0.5 and 1 mg/mL of nanoparticles were produced HSA with intact structure. But immobilization with 2 mg/mL of nanoparticles resulted in some modifications in the secondary structures (i.e., α-helixes and β-turns) of produced HSA. Based on these data, immobilization of P. pastoris cells with 0.5 or 1 mg/mL of nanoparticles is completely efficient for cell harvesting and has any effect on the structure of recombinant product. These findings revealed that decoration of microbial cells with high concentrations of nanoparticles has some impacts on the structure of secretory proteins.












Similar content being viewed by others

References
Lu, J., Peng, W., Lv, Y., Jiang, Y., Xu, B., Zhang, W., et al. (2020). Application of cell immobilization technology in microbial cocultivation systems for biochemicals production. Industrial and Engineering Chemistry Research, 59, 17026–17034.
Bouabidi, Z. B., El-Naas, M. H., & Zhang, Z. (2019). Immobilization of microbial cells for the biotreatment of wastewater: A review. Environmental Chemistry Letters, 17, 241–257.
Trelles, J. A., & Rivero, C. W. (2020). Whole cell entrapment techniques. In J. M. Guisan, J. Bolivar, F. López-Gallego, & J. Rocha-Martín (Eds.), Immobilization of enzymes and cells (Vol. 2100, pp. 385–394). New York: Humana.
Abarian, M., Hassanshahian, M., & Esbah, A. (2019). Degradation of phenol at high concentrations using immobilization of Pseudomonas putida P53 into sawdust entrapped in sodium-alginate beads. Water Science and Technology, 79, 1387–1396.
Terpou, A., Ganatsios, V., Kanellaki, M., & Koutinas, A. A. (2020). Entrapped psychrotolerant yeast cells within pine sawdust for low temperature wine making: Impact on wine quality. Microorganisms, 8, 764.
Chen, C., Hu, J., & Wang, J. (2020). Uranium biosorption by immobilized active yeast cells entrapped in calcium-alginate-PVA-GO-crosslinked gel beads. Radiochimica Acta, 108, 273–286.
Göksungur, Y., & Zorlu, N. (2001). Production of ethanol from beet molasses by Ca-alginate immobilized yeast cells in a packed-bed bioreactor. Turk. J. Biol., 25, 265–275.
Thipayarat, A. (2003). Production of human serum albumin by immobilized yeast. Ph.D. Thesis, Syracuse University, Syracuse, USA.
Berovic, M., Berlot, M., Kralj, S., & Makovec, D. (2014). A new method for the rapid separation of magnetized yeast in sparkling wine. Biochemical Engineering Journal, 88, 77–84.
Safarik, I., & Safarikova, M. (2007). Magnetically modified microbial cells: A new type of magnetic adsorbents. China Particuology, 5, 19–25.
Safarik, I., Maderova, Z., Pospiskova, K., Baldikova, E., Horska, K., & Safarikova, M. (2015). Magnetically responsive yeast cells: Methods of preparation and applications. Yeast, 32, 227–237.
Ebrahiminezhad, A., Taghizadeh, S.-M., Ghasemi, Y., & Berenjian, A. (2020). Immobilization of cells by magnetic nanoparticles. In J. M. Guisan, J. M. Bolivar, F. López-Gallego, & J. Rocha-Martín (Eds.), Immobilization of enzymes and cells (Vol. 2100, pp. 427–435). New York: Humana.
Safarik, I., Pospiskova, K., Maderova, Z., Baldikova, E., Horska, K., & Safarikova, M. (2015). Microwave-synthesized magnetic chitosan microparticles for the immobilization of yeast cells. Yeast, 32, 239–243.
Ansari, F., Grigoriev, P., Libor, S., Tothill, I. E., & Ramsden, J. J. (2009). DBT degradation enhancement by decorating Rhodococcus erythropolis IGST8 with magnetic Fe3O4 nanoparticles. Biotechnology and Bioengineering, 102, 1505–1512.
Raee, M. J., Ebrahiminezhad, A., Gholami, A., Ghoshoon, M. B., & Ghasemi, Y. (2018). Magnetic immobilization of recombinant E. coli producing extracellular asparaginase: An effective way to intensify downstream process. Separation Science and Technology, 53, 1397–1404.
Taghizadeh, S.-M., Jafari, S., Ahmad-Kiadaliri, T., Mobasher, M. A., Lal, N., Raee, M. J., et al. (2019). Magnetic immobilisation as a promising approach against bacteriophage infection. Materials Research Express, 6, 1250–1258.
Taghizadeh, S.-M., Ebrahiminezhad, A., Ghoshoon, M. B., Dehshahri, A., Berenjian, A., & Ghasemi, Y. (2020). Magnetic immobilization of Pichia pastoris cells for the production of recombinant human serum albumin. Nanomaterials, 10, 111.
Ebrahiminezhad, A., Varma, V., Yang, S., & Berenjian, A. (2016). Magnetic immobilization of Bacillus subtilis natto cells for menaquinone-7 fermentation. Applied Microbiology and Biotechnology, 100, 173–180.
Ebrahiminezhad, A., Varma, V., Yang, S., Ghasemi, Y., & Berenjian, A. (2015). Synthesis and application of amine functionalized iron oxide nanoparticles on menaquinone-7 fermentation: A step towards process intensification. Nanomaterials, 6, 1.
Taghizadeh, S. M., Berenjian, A., Chew, K. W., Show, P. L., Mohd Zaid, H. F., Ramezani, H., et al. (2020). Impact of magnetic immobilization on the cell physiology of green unicellular algae Chlorella vulgaris. Bioengineered, 11, 141–153.
Daly, R., & Hearn, M. T. (2005). Expression of heterologous proteins in Pichia pastoris: A useful experimental tool in protein engineering and production. Journal of Molecular Recognition, 18, 119–138.
Lima-Pérez, J., Rodríguez-Gómez, D., Loera, O., Viniegra-González, G., & López-Pérez, M. (2018). Differences in growth physiology and aggregation of Pichia pastoris cells between solid-state and submerged fermentations under aerobic conditions. Journal of Chemical Technology and Biotechnology, 93, 527–532.
Doaga, R., McCormac, T., & Dempsey, E. (2020). Functionalized magnetic nanomaterials for electrochemical biosensing of cholesterol and cholesteryl palmitate. Microchimica Acta, 187, 225.
Kaushik, N., Lamminmäki, U., Khanna, N., & Batra, G. (2020). Enhanced cell density cultivation and rapid expression-screening of recombinant Pichia pastoris clones in microscale. Scientific Reports, 10, 7458.
Olson, B. J., & Markwell, J. (2007). Assays for determination of protein concentration (p. 48). Pharmacol: Curr. Protoc.
Yang, Q., Liang, J., & Han, H. (2009). Probing the interaction of magnetic iron oxide nanoparticles with bovine serum albumin by spectroscopic techniques. The Journal of Physical Chemistry B, 113, 10454–10458.
Usoltsev, D., Sitnikova, V., Kajava, A., & Uspenskaya, M. (2019). Systematic FTIR spectroscopy study of the secondary structure changes in human serum albumin under various denaturation conditions. Biomolecules, 9, 359.
Ebrahiminezhad, A., Ghasemi, Y., Rasoul-Amini, S., Barar, J., & Davaran, S. (2013). Preparation of novel magnetic fluorescent nanoparticles using amino acids. Colloids and Surfaces B, 102, 534–539.
Rosenholm, J. M., Zhang, J., Sun, W., & Gu, H. (2011). Large-pore mesoporous silica-coated magnetite core-shell nanocomposites and their relevance for biomedical applications. Microporous and Mesoporous Materials, 145, 14–20.
Pham, X. N., Nguyen, T. P., Pham, T. N., Tran, T. T. N., & Tran, T. V. T. (2016). Synthesis and characterization of chitosan-coated magnetite nanoparticles and their application in curcumin drug delivery. Advances in Natural Sciences: Nanoscience and Nanotechnology, 7, 045010.
Li, Y. G., Gao, H. S., Li, W. L., Xing, J. M., & Liu, H. Z. (2009). In situ magnetic separation and immobilization of dibenzothiophene-desulfurizing bacteria. Bioresource Technology, 100, 5092–5096.
Aghili, Z., Taheri, S., Zeinabad, H. A., Pishkar, L., Saboury, A. A., Rahimi, A., et al. (2016). Investigating the interaction of Fe nanoparticles with lysozyme by biophysical and molecular docking studies. PLoS ONE, 11, e0164878.
Varkhede, N., Peters, B.-H., Wei, Y., Middaugh, C. R., Schöneich, C., & Forrest, M. L. (2019). Effect of iron oxide nanoparticles on the oxidation and secondary structure of growth hormone. Journal of Pharmaceutical Sciences, 108, 3372–3381.
Vakili-Ghartavol, R., Momtazi-Borojeni, A. A., Vakili-Ghartavol, Z., Aiyelabegan, H. T., Jaafari, M. R., Rezayat, S. M., et al. (2020). Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artificial Cells, Nanomedicine, and Biotechnology, 48, 443–451.
Malhotra, N., Lee, J.-S., Liman, R. A. D., Ruallo, J. M. S., Villaflores, O. B., Ger, T.-R., et al. (2020). Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules, 25, 3159.
Simonsen, G., Strand, M., & Øye, G. (2018). Potential applications of magnetic nanoparticles within separation in the petroleum industry. Journal of Petroleum Science and Engineering, 165, 488–495.
Antarnusa, G., & Suharyadi, E. (2020). A synthesis of polyethylene glycol (PEG)-coated magnetite Fe3O4 nanoparticles and their characteristics for enhancement of biosensor. Materials Research Express, 7, 056103.
Lazaro-Carrillo, A., Filice, M., Guillén, M. J., Amaro, R., Viñambres, M., Tabero, A., et al. (2020). Tailor-made PEG coated iron oxide nanoparticles as contrast agents for long lasting magnetic resonance molecular imaging of solid cancers. Materials Science and Engineering C, 107, 110262.
Pham, X.-H., Hahm, E., Kim, H.-M., Son, B. S., Jo, A., An, J., et al. (2020). Silica-coated magnetic iron oxide nanoparticles grafted onto graphene oxide for protein isolation. Nanomaterials, 10, 117.
Acknowledgements
This experiment was financially supported by Shiraz University of Medical Sciences as PhD thesis proposal of Seyedeh-Masoumeh Taghizadeh submitted at No. 18588 in the School of Pharmacy. Authors are grateful to the support provided by the University of Waikato, New Zealand.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest with the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taghizadeh, SM., Ghoshoon, M.B., Berenjian, A. et al. Impacts of Magnetic Immobilization on the Recombinant Proteins Structure Produced in Pichia pastoris System. Mol Biotechnol 63, 80–89 (2021). https://doi.org/10.1007/s12033-020-00286-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-020-00286-4