Abstract
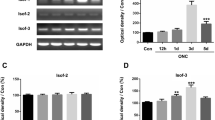
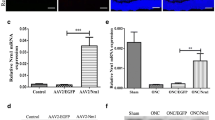
To investigate the functional role of fasudil in optic nerve crush (ONC), and further explore its possible molecular mechanism. After ONC injury, the rats were injected intraperitoneally either with fasudil or normal saline once a day until euthanized. RGCs survival was assessed by retrograde labeling with FluoroGold. Retinal glial cells activation and population changes (GFAP, iba-1) were measured by immunofluorescence. The expressions of cleaved caspase 3 and 9, p-ERK1/2 and p-AKT were detected by western blot. The levels of the pro-inflammatory cytokines were determined using real-time polymerase chain reaction. Fasudil treatment inhibited RGCs apoptosis and reduced RGCs loss demonstrated by the decreased apoptosis-associated proteins expression and the increased fluorogold labeling of RGCs after ONC, respectively. In addition, the ONC + fasudil group compared had a significantly lower expression of GFAP and iba1 compared with the ONC group. The levels of pro-inflammatory cytokines were significantly reduced in the ONC + fasudil group than in the ONC group. Furthermore, the phosphorylation levels of ERK1/2 and AKT (p-ERK1/2 and p-AKT) were obviously elevated by the fasudil treatment. Our study demonstrated that fasudil attenuated glial cell-mediated neuroinflammation by up-regulating the ERK1/2 and AKT signaling pathways in rats ONC models. We conclude that fasudil may be a novel treatment for traumatic optic neuropathy.





Similar content being viewed by others
References
Chen M, Jiang Y, Zhang J, Li N (2018) Clinical treatment of traumatic optic neuropathy in children: summary of 29 cases. Exp Ther Med 16:3562–3566. https://doi.org/10.3892/etm.2018.6637
Kashkouli MB, Yousefi S, Nojomi M, Sanjari MS, Pakdel F, Entezari M, Etezad-Razavi M, Razeghinejad MR, Esmaeli M, Shafiee M, Bagheri M (2018) Traumatic optic neuropathy treatment trial (TONTT): open label, phase 3, multicenter, semi-experimental trial. Graefes Arch Clin Exp Ophthalmol 256:209–218. https://doi.org/10.1007/s00417-017-3816-5
Lee WJ, Hong EH, Park HM, Lim HW (2019) Traumatic optic neuropathy-associated progressive thinning of the retinal nerve fiber layer and ganglion cell complex: two case reports. BMC Ophthalmol 19:216. https://doi.org/10.1186/s12886-019-1232-9
Yu J, Lan S, Wang R, Maier A (2015) Fasudil alleviates traumatic optic neuropathy by inhibiting Rho signaling pathway. Int J Clin Exp Med 8:13377–13382
Yu J, Lin L, Luan X, Jing X et al (2015) Impacts of Rho kinase inhibitor Fasudil on Rho/ROCK signaling pathway in rabbits with optic nerve injury. Int J Clin Exp Pathol 8:14717–14724
Yu J, Lan S, Wang R, Maier A, Luan X (2015) Fasudil alleviates traumatic optic neuropathy by inhibiting Rho signaling pathway. Int J Clin Exp Med 8:13377–13382
Yu J, Luan X, Lan S, Yan B, Maier A (2016) Fasudil, a rho-associated protein kinase inhibitor, attenuates traumatic retinal nerve injury in rabbits. J Mol Neurosci 58:74–82. https://doi.org/10.1007/s12031-015-0691-6
Rovere G, Nadal-Nicolas FM, Sobrado-Calvo P, Garcia-Bernal D, Villegas-Perez MP, Vidal-Sanz M, Agudo-Barriuso M (2016) Topical treatment with bromfenac reduces retinal gliosis and inflammation after optic nerve crush. Invest Ophthalmol Vis Sci 57:6098–6106. https://doi.org/10.1167/iovs.16-20425
Bond WS, Rex TS (2014) Evidence that erythropoietin modulates neuroinflammation through differential action on neurons, astrocytes, and microglia. Front Immunol 5:523. https://doi.org/10.3389/fimmu.2014.00523
Cueva Vargas JL, Belforte N, Di Polo A (2016) The glial cell modulator ibudilast attenuates neuroinflammation and enhances retinal ganglion cell viability in glaucoma through protein kinase A signaling. Neurobiol Dis 93:156–171. https://doi.org/10.1016/j.nbd.2016.05.002
Jia Y, Jiang S, Chen C, Lu G, Xie Y, Sun X, Huang L (2019) Caffeic acid phenethyl ester attenuates nuclear factor kappaB mediated inflammatory responses in Muller cells and protects against retinal ganglion cell death. Mol Med Rep 19:4863–4871. https://doi.org/10.3892/mmr.2019.10151
Mac Nair CE, Schlamp CL, Montgomery AD, Shestopalov VI, Nickells RW (2016) Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J Neuroinflamm 13:93. https://doi.org/10.1186/s12974-016-0558-y
Adornetto A, Russo R, Parisi V (2019) Neuroinflammation as a target for glaucoma therapy. Neural Regen Res 14:391–394. https://doi.org/10.4103/1673-5374.245465
Welser-Alves JV, Milner R (2013) Microglia are the major source of TNF-alpha and TGF-beta1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem Int 63:47–53. https://doi.org/10.1016/j.neuint.2013.04.007
He D, Huang B, Fu S, Li Y, Ran X, Liu Y, Chen G, Liu J, Liu D (2018) Tubeimoside I protects dopaminergic neurons against inflammation-mediated damage in lipopolysaccharide (LPS)-evoked model of Parkinson’s disease in rats. Int J Mol Sci 19:2242. https://doi.org/10.3390/ijms19082242
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97. https://doi.org/10.1186/1479-5876-7-97
Wu N, Yu J, Chen S, Xu J, Ying X, Ye M, Li Y, Wang Y (2014) αCrystallin protects RGC survivaland inhibits microglial activation after optic nerve crush. Life Sci 94:17–23. https://doi.org/10.1016/j.lfs.2013.10.034
McGeer PL, McGeer EG (1995) The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev 21:195–218. https://doi.org/10.1016/0165-0173(95)00011-9
Tsai RK, Chang CH, Wang HZ (2008) Neuroprotective effects of recombinant human granulocyte colony-stimulating factor (G-CSF) in neurodegeneration after optic nerve crush in rats. Exp Eye Res 87:242–250. https://doi.org/10.1016/j.exer.2008.06.004
Levkovitch-Verbin H, Waserzoog Y, Vander S, Makarovsky D, Piven I (2014) Minocycline upregulates pro-survival genes and downregulates pro-apoptotic genes in experimental glaucoma. Graefes Arch Clin Exp Ophthalmol 252:761–772. https://doi.org/10.1007/s00417-014-2588-4
Yang X, Hondur G, Tezel G (2016) Antioxidant treatment limits neuroinflammation in experimental glaucoma. Investig Opthalmol Vis Sci. https://doi.org/10.1167/iovs.16-19153
Xu P, Huang MW, Xiao CX, Long F, Wang Y, Liu SY, Jia WW, Wu WJ, Yang D, Hu JF, Liu XH, Zhu YZ (2017) Matairesinol suppresses neuroinflammation and migration associated with Src and ERK1/2-NF-kappaB pathway in activating BV2 microglia. Neurochem Res 42:2850–2860. https://doi.org/10.1007/s11064-017-2301-1
Fujita Y, Sato A, Yamashita T (2013) Brimonidine promotes axon growth after optic nerve injury through Erk phosphorylation. Cell Death Dis 4:e763. https://doi.org/10.1038/cddis.2013.298
Kaplan DR, Miller FD (2000) Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10:381–391. https://doi.org/10.1016/s0959-4388(00)00092-1
Kermer P, Klöcker N, Labes M, Bähr M (2000) Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci 20:2–8
Li DJ, Li YH, Yuan HB, Qu LF, Wang P (2017) The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 68:31–42. https://doi.org/10.1016/j.metabol.2016.12.003
Pernet V, Joly S, Jordi N, Dalkara D, Guzik-Kornacka A, Flannery JG, Schwab ME (2013) Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis 4:e734. https://doi.org/10.1038/cddis.2013.266
Kwon JH, Gaire BP, Park SJ, Shin DY, Choi JW (2018) Identifying lysophosphatidic acid receptor subtype 1 (LPA1) as a novel factor to modulate microglial activation and their TNF-alpha production by activating ERK1/2. Biochim Biophys Acta Mol Cell Biol Lipids 1863:1237–1245. https://doi.org/10.1016/j.bbalip.2018.07.015
Gaire BP, Song MR, Choi JW (2018) Sphingosine 1-phosphate receptor subtype 3 (S1P3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their M1 polarization. J Neuroinflamm 15:284. https://doi.org/10.1186/s12974-018-1323-1
Huang C, Lu X, Wang JL, Tong LJ, Ling Y, Jiang B, Yang RR, Zhang W (2016) Compound C induces the ramification of murine microglia in an AMPK-independent and small rhogtpase-dependent manner. Neuroscience 331:24–39. https://doi.org/10.1016/j.neuroscience.2016.06.018
Chang YC, Lin CY, Hsu CM, Lin HC, Chen YH, Lee-Chen GJ, Su MT, Ro LS, Chen CM, Hsieh-Li HM (2011) Neuroprotective effects of granulocyte-colony stimulating factor in a novel transgenic mouse model of SCA17. J Neurochem 118:288–303. https://doi.org/10.1111/j.1471-4159.2011.07304
Tsai RK, Chang CH, Sheu MM, Huang ZL (2010) Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Exp Eye Res 90:537–545. https://doi.org/10.1016/j.exer.2010.01.004
Hara M, Takayasu M, Watanabe K, Noda A, Takagi T, Suzuki Y, Yoshida J (2000) Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J Neurosurg 93:94–101
Shintani N, Ishiyama T, Kotoda M, Asano N, Sessler DI, Matsukawa T (2017) The effects of Y-27632 on pial microvessels during global brain ischemia and reperfusion in rabbits. BMC Anesthesiol 17:38. https://doi.org/10.1186/s12871-017-0331-5
Wu J, Li J, Hu H, Liu P, Fang Y, Wu D (2012) Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell Mol Neurobiol 32:1187–1197. https://doi.org/10.1007/s10571-012-9845-z
Chen M, Liu A, Ouyang Y, Huang Y, Chao X, Pi R (2013) Fasudil and its analogs: a new powerful weapon in the long war against central nervous system disorders? Expert Opin Investig Drugs 22:537–550. https://doi.org/10.1517/13543784.2013.778242
Ruan H, Zhang Y, Liu R, Yang X (2019) The acute effects of 30 mg vs 60 mg of intravenous Fasudil on patients with congenital heart defects and severe pulmonary arterial hypertension. Congenit Heart Dis 14:645–650. https://doi.org/10.1111/chd.12764
Zhang Y, Wu S (2017) Effects of fasudil on pulmonary hypertension in clinical practice. Pulm Pharmacol Ther 46:54–63. https://doi.org/10.1016/j.pupt.2017.08.002
Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A (2002) Suppression of coronary artery spasm by the rho-kinase inhibitor fasudil in patients with V asospastic angina. Circulation 105:1545–1547. https://doi.org/10.1161/hc1302.105938
Zhao J, Zhou D, Guo J, Ren Z, Zhou L, Wang S, Xu B, Wang R (2006) Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 46:421–428
Sugiyama T, Shibata M, Kajiura S, Okuno T, Tonari M, Oku H, Ikeda T (2011) Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits. Invest Ophthalmol Vis Sci 52:64–69. https://doi.org/10.1167/iovs.10-5265
Li Q, Huang XJ, He W, Ding J, Jia JT, Fu G, Wang HX, Guo LJ (2009) Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats. Cell Mol Neurobiol 29:169–180. https://doi.org/10.1007/s10571-008-9308-8
Ichikawa M, Yoshida J, Saito K, Sagawa H, Tokita Y, Watanabe M (2008) Differential effects of two ROCK inhibitors, Fasudil and Y-27632, on optic nerve regeneration in adult cats. Brain Res 1201:23–33. https://doi.org/10.1016/j.brainres.2008.01.063
Brunet A, Datta SR, Greenberg ME (2011) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11:297–305
Ahmed LA, Darwish HA, Abdelsalam RM, Amin HA (2016) Role of rho kinase inhibition in the protective effect of fasudil and simvastatin against 3-nitropropionic acid-induced striatal neurodegeneration and mitochondrial dysfunction in rats. Mol Neurobiol 53:3927–3938. https://doi.org/10.1007/s12035-015-9303-2
Fu PC, Tang RH, Yu ZY, Xie MJ, Wang W, Luo X (2018) The Rho-associated kinase inhibitors Y27632 and fasudil promote microglial migration in the spinal cord via the ERK signaling pathway. Neural Regen Res 13:677–683. https://doi.org/10.4103/1673-5374.230294
Takata M, Tanaka H, Kimura M, Nagahara Y, Tanaka K, Kawasaki K, Seto M, Tsuruma K, Shimazawa M, Hara H (2013) Fasudil, a rho kinase inhibitor, limits motor neuron loss in experimental models of amyotrophic lateral sclerosis. Br J Pharmacol 170:341–351. https://doi.org/10.1111/bph.12277
Tönges L, Frank T, Tatenhorst L, Saal KA, Koch JC, Szego EM, Bahr M, Weishaupt JH, Lingor P (2012) Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson’s disease. Brain 135:3355–3370. https://doi.org/10.1093/brain/aws254
Wu N, Li W, Shu W, Lv Y, Jia D (2014) Inhibition of Rho-kinase by fasudil restores the cardioprotection of ischemic postconditioninng in hypercholesterolemic rat heart. Mol Med Rep 10:2517–2524. https://doi.org/10.3892/mmr.2014.2566
Wu Y, Xu F, Huang H, Chen L, Wen M, Jiang L, Lu L, Li L, Song D, Zeng S, Li L, Li M (2014) Up-regulation of SKIP relates to retinal ganglion cells apoptosis after optic nerve crush in vivo. J Mol Histol 45:715–721. https://doi.org/10.1007/s10735-014-9589-9
He Q, Li YH, Guo SS, Wang Y, Lin W, Zhang Q, Wang J, Ma CG, Xiao BG (2016) Inhibition of Rho-kinase by Fasudil protects dopamine neurons and attenuates inflammatory response in an intranasal lipopolysaccharide-mediated Parkinson’s model. Eur J Neurosci 43:41–52
Zhang H, Li Y, Yu J, Guo M, Meng J, Liu C, Xie Y, Feng L, Xiao B, Ma C (2013) Rho kinase inhibitor fasudil regulates microglia polarization and function. Neuroimmunomodulation 20:313–322. https://doi.org/10.1159/000351221
Huang R, Lan Q, Chen L, Zhong H, Cui L, Jiang L, Huang H, Li L, Zeng S, Li M, Zhao X, Xu F (2018) CD200Fc attenuates retinal glial responses and RGCs apoptosis after optic nerve crush by modulating CD200/CD200R1 interaction. J Mol Neurosci 64:200–210. https://doi.org/10.1007/s12031-017-1020-z
Tang Z, Zhang S, Lee C, Kumar A, Arjunan P, Li Y, Zhang F, Li X (2011) An optic nerve crush injury murine model to study retinal ganglion cell survival. J Vis Exp. https://doi.org/10.3791/2685
Xu F, Huang H, Wu Y, Lu L, Jiang L, Chen L, Zeng S, Li L, Li M (2014) Upregulation of Gem relates to retinal ganglion cells apoptosis after optic nerve crush in adult rats. J Mol Histol 45:565–571. https://doi.org/10.1007/s10735-014-9579-y
Xu Y, Yang B, Hu Y, Lu L, Lu X, Wang J, Xu F, Yu S, Huang J (2016) Wogonin prevents TLR4-NF-κB-medicated neuro-inflammation and improves retinal ganglion cells survival in retina after optic nerve crush. Oncotarget 7:72503–72517
Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ (1994) Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 14:4368–4374
Wang J, Chen S, Zhang X, Huang W, Jonas JB (2016) Intravitreal triamcinolone acetonide, retinal microglia and retinal ganglion cell apoptosis in the optic nerve crush model. Acta Ophthalmol 94:e305-311. https://doi.org/10.1111/aos.12698
Wang R, Sun Q, Xia F, Chen Z, Wu J, Zhang Y, Xu J, Liu L (2017) Methane rescues retinal ganglion cells and limits retinal mitochondrial dysfunction following optic nerve crush. Exp Eye Res 159:49–57. https://doi.org/10.1016/j.exer.2017.03.008
Wang R, Xu J, Xie J, Kang Z, Sun X, Chen N, Liu L, Xu J (2010) Hyperbaric oxygen preconditioning promotes survival of retinal ganglion cells in a rat model of optic nerve crush. J Neurotrauma 27:763–770
Zhong H, Cui L, Xu F, Chen L, Jiang L, Huang H, Xu J, Zhao X, Li L, Zeng S, Li M (2016) Up-regulation of Wip1 involves in neuroinflammation of retinal astrocytes after optic nerve crush via NF-kappaB signaling pathway. Inflamm Res 65:709–715. https://doi.org/10.1007/s00011-016-0952-z
Brockmann C, Corkhill C, Jaroslawska E, Dege S, Brockmann T, Kociok N, Joussen AM (2019) Systemic Rho-kinase inhibition using fasudil in mice with oxygen-induced retinopathy. Graefes Arch Clin Exp Ophthalmol 257:1699–1708. https://doi.org/10.1007/s00417-019-04365-4
Zhu WW, Ma XL, Guo AL, Zhao HY, Luo HH (2011) Neuroprotective effects of NEP1-40 and fasudil on Nogo-A expression in neonatal rats with hypoxic-ischemic brain damage. Genet Mol Res 10:2987–2995. https://doi.org/10.4238/2011.November.29.9
Huang TL, Huang SP, Chang CH, Lin KH, Sheu MM, Tsai RK (2014) Factors influencing the retrograde labeling of retinal ganglion cells with fluorogold in an animal optic nerve crush model. Ophthalmic Res 51:173–178. https://doi.org/10.1159/000357736
Koeberle PD, Ball AK (2002) Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience 110:555–567
Tsuda S, Tanaka Y, Kunikata H, Yokoyama Y, Yasuda M, Ito A, Nakazawa T (2016) Real-time imaging of RGC death with a cell-impermeable nucleic acid dyeing compound after optic nerve crush in a murine model. Exp Eye Res 146:179–188. https://doi.org/10.1016/j.exer.2016.03.017
Yamamoto K, Maruyama K, Himori N, Omodaka K, Yokoyama Y, Shiga Y, Morin R, Nakazawa T (2014) The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest Ophthalmol Vis Sci 55:7126–7136. https://doi.org/10.1167/iovs.13-13842
Zhang ZZ, Gong YY, Shi YH, Zhang W, Qin XH, Wu XW (2012) V alproate promotes survival of retinal ganglion cells in a rat model of optic nerve crush. Neuroscience 224:282–293. https://doi.org/10.1016/j.neuroscience.2012.07.056
Ma K, Xu L, Zhang H, Zhang S, Pu M, Jonas JB (2009) Effect of brimonidine on retinal ganglion cell survival in an optic nerve crush model. Am J Ophthalmol 147:326–331. https://doi.org/10.1016/j.ajo.2008.08.005
Zhang R, Zhang H, Xu L, Ma K, Wallrapp C, Jonas JB (2011) Neuroprotective effect of intravitreal cell-based glucagon-like peptide-1 production in the optic nerve crush model. Acta Ophthalmol 89:e320–e326. https://doi.org/10.1111/j.1755-3768.2010.02044
Cui L, He WJ, Xu F, Jiang L, Lv ML, Huang H, Xu JP, Wu Y, Zhong HB, Zhang SY, Chen LF, Shen CL, Yao G, Li L, Li M, Zeng SM (2016) Alterations in the expression of Hs1-associated protein X-1 in the rat retina after optic nerve crush. Mol Med Rep 14:4761–4766. https://doi.org/10.3892/mmr.2016.5824
Jiang L, Xu F, He W, Chen L, Zhong H, Wu Y, Zeng S, Li L, Li M (2016) CD200Fc reduces TLR4-mediated inflammatory responses in LPS-induced rat primary microglial cells via inhibition of the NF-kappaB pathway. Inflamm Res 65:521–532. https://doi.org/10.1007/s00011-016-0932-3
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Song H, Gao D (2011) Fasudil, a Rho-associated protein kinase inhibitor, attenuates retinal ischemia and reperfusion injury in rats. Int J Mol Med 28:193–198
Lu XC, Shear DA, Graham PB, Bridson GW, Uttamsingh V, Chen Z, Leung LY, Tortella FC (2015) Dual therapeutic effects of C-10068, a dextromethorphan derivative, against post-traumatic nonconvulsive seizures and neuroinflammation in a rat model of penetrating ballistic-like brain injury. J Neurotrauma 32:1621–1632. https://doi.org/10.1089/neu.2014.3766
Luo JM, Cen LP, Zhang XM, Chiang SW, Huang Y, Lin D, Fan YM, van Rooijen N, Lam DS, Pang CP, Cui Q (2007) PI3K/akt, JAK/STA T and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci 26:828–842. https://doi.org/10.1111/j.1460-9568.2007.05718.x
Hannan JL, Matsui H, Sopko NA, Liu X, Weyne E, Albersen M, Watson JW, Hoke A, Burnett AL, Bivalacqua TJ (2016) Caspase-3 dependent nitrergic neuronal apoptosis following cavernous nerve injury is mediated via RhoA and ROCK activation in major pelvic ganglion. Sci Rep 6:29416. https://doi.org/10.1038/srep29416
Cheung ZH, Chan YM, Siu FK, Yip HK, Wu W, Leung MC, So KF (2004) Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci 25:383–393. https://doi.org/10.1016/j.mcn.2003.11.001
Kermer P, Ankerhold R, Klöcker N, Krajewski S, Reed JC, Bähr M (2000) Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res 85:144–150
Kermer P, Klöcker N, Labes M, Thomsen S, Srinivasan A, Bähr M (1999) Activation of caspase-3 in axotomized rat retinal ganglion cells in vivo. FEBS Lett 453:361–364
Ye D, Shi Y, Xu Y, Huang J (2019) PACAP attenuates optic nerve crush-induced retinal ganglion cell apoptosis via activation of the CREB-Bcl-2 pathway. J Mol Neurosci 68:475–484. https://doi.org/10.1007/s12031-019-01309-9
Kermer P, Klöcker N, Labes M, Bähr M (1998) Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci 18:4656–4662
Liu Y, Yan H, Chen S, Sabel BA (2015) Caspase-3 inhibitor Z-DEVD-FMK enhances retinal ganglion cell survival and vision restoration after rabbit traumatic optic nerve injury. Restor Neurol Neurosci 33:205–220. https://doi.org/10.3233/RNN-159001
Lu YB, Pannicke T, Wei EQ, Bringmann A, Wiedemann P, Habermann G, Buse E, Käs JA, Reichenbach A (2013) Biomechanical properties of retinal glial cells: comparative and developmental data. Exp Eye Res 113:60–65. https://doi.org/10.1016/j.exer.2013.05.012
Rosen AM, Stevens B (2010) The role of the classical complement cascade in synapse loss during development and glaucoma. Adv Exp Med Biol 703:75–93. https://doi.org/10.1007/978-1-4419-5635-4_6
Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G (2008) Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol 86:398–408. https://doi.org/10.1038/icb.2008.19
Zhao Y, Zhang Q, Xi J, Xiao B, Li Y, Ma C (2015) Neuroprotective effect of fasudil on inflammation through PI3K/Akt and Wnt/beta-catenin dependent pathways in a mice model of Parkinson’s disease. Int J Clin Exp Pathol 8:2354–2364
Xueyang D, Zhanqiang M, Chunhua M, Kun H (2016) Fasudil, an inhibitor of Rho-associated coiled-coil kinase, improves cognitive impairments induced by smoke exposure. Oncotarget 7:78764–78772
Johnson K, D’Mello SR (2005) p21-Activated kinase-1 is necessary for depolarization-mediated neuronal survival. J Neurosci Res 79:809–815. https://doi.org/10.1002/jnr.20415
Marra C, Gomes Moret D, de Souza CA, Chagas da Silva F, Moraes P, Linden R, Sholl-Franco A (2011) Protein kinases JAK and ERK mediate protective effect of interleukin-2 upon ganglion cells of the developing rat retina. J Neuroimmunol 233:120–126. https://doi.org/10.1016/j.jneuroim.2010.12.008
Pernet V, Hauswirth WW, Di Polo A (2005) Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo. J Neurochem 93:72–83. https://doi.org/10.1111/j.1471-4159.2005.03002.x
Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UAK, Müller W, Musiani P, Poli V, Davies AM (2001) Role of STA T3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci 18:270–282. https://doi.org/10.1006/mcne.2001.1018
Acknowledgements
Chinese government provided financial support in the form of the National Natural Science Foundation of China (Grant Nos. 81560166, 81460087, 81660168, 81660161, 8176040227 and 81760172) and the Natural Science Foundation of Guangxi Zhuang Autonomous Region (Grant Nos. 2018GXNSFAA281128 and 2018GXNSFBA281066).
Funding
This study was supported by the National Natural Science Foundation of China (No. 81660161, 81560166, 81460087, 81660168, 8176040227 and 81760172), and the Natural Science Foundation of Guangxi Zhuang Autonomous Region (No. 2018GXNSFAA281128 and No. 2018GXNSFBA281066) and Guangxi clinical ophthalmic research center (No. AD19245193).
Author information
Authors and Affiliations
Contributions
QL, LJ and FX conceived and designed the experiments. ZM, BH, NL and MZ performed the establishment of model and the intraperitoneal injection. The retrograde labeling of RGCs with FluoroGold was performed by WH, HH and JL. WH, LJ and WY performed the western blot analysis and immunofluorescence staining experiments. FT, CS and JL performed the qPCR. WH, QL, LJ, FT analyzed and interpreted the data. WH, QL, LJ, and LC edited the paper. HZ, SZ, ML, LC and FX read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, W., Lan, Q., Jiang, L. et al. Fasudil attenuates glial cell-mediated neuroinflammation via ERK1/2 and AKT signaling pathways after optic nerve crush. Mol Biol Rep 47, 8963–8973 (2020). https://doi.org/10.1007/s11033-020-05953-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05953-y