Abstract
Purpose
The industrial applications of lipases are hampered by their sensitive nature to processing and storage conditions. In this context, this study presents a simple statistical modeling approach using Nano Spray Dryer BÜCHI B-90 as an ideal technique for the production of stable lipase nano-powder (LNP).
Methods
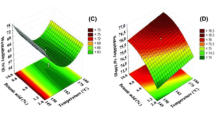
In this study, factorial design is employed to optimize the spray drying process for Mucor racemosus lipase enzyme. Different variables were investigated such as adjuvant type (dextrin, mannitol, and lactose), concentration (2%, 4%, 8%), nozzle diameter, and inlet temperature (80 °C, 100 °C, 120 °C). Additionally, the physicochemical properties of LNP including particle size, residual enzyme activity, powder yield, morphology, and moisture content are investigated.
Results
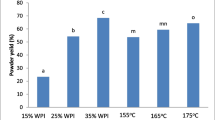
Results indicate a significant effect of additive type on product yield value and particle size. LNP exhibited a broad range of physical characteristics (enzyme residual activity 80.5–100%, particle size 210–1313 nm, and yield value 24–99%) and 98.4% residual activity after storage for 2 months at 5 °C. Scanning electron microscopy (SEM) images have shown a variation in the morphology of LNP with a remarkable flower-like structure in presence of lactose. The measured pronounced lipase stability is accredited to high local lipase concentration, adjuvant stabilizing effect, and favorable lipase conformation at the nano-scale-solid state.
Conclusion
Based on the results presented, a robust approach for the production of a maximally viable spray-dried LNP in solid form can be employed for extended biotherapeutic and biotechnological applications of lipase.





Similar content being viewed by others
References
Loli H, Narwal SK, Saun NK, Gupta R. Lipases in medicine: an overview. Mini-Rev Med Chem. 2015;15(14):1209–16.
Mehta A, Bodh U, Gupta R. Fungal lipases: a review. J Biotech Res. 2017;8:58–77.
Choi J-M, Han S-S, Kim H-S. Industrial applications of enzyme biocatalysis: current status and future aspects. Biotechnol Adv. 2015;33:1443–54.
Mohamed SA, Abdel-Mageed HM, Tayel SA. Characterization of Mucor racemosus lipase with potential application for the treatment of cellulite. Process Biochem. 2011;46:642–8.
Pulat M, Akalin G. Preparation and characterization of gelatin hydrogel support for immobilization of Candida Rugosa lipase. Artif Cells Nanomed Biotechnol. 2013;41:145–51. https://doi.org/10.3109/10731199.2012.696070.
Anobom C, Pinheiro A, De-Andrade R, et al. From structure to catalysis: recent developments in the biotechnological applications of lipases. Biomed Res Int. 2014;684506:1–11. https://doi.org/10.1155/2014/684506.
Abdel-Mageed HM, El-Laithy H, Mahran L, Mohamed SA, Fahmy A, Mäder K. Development of novel flexible sugar ester vesicles as carrier systems for antioxidant catalase enzyme for wound healing application. Process Biochem. 2012;47:1155–62.
Abdel-Mageed HM, Radwan R, AbuelEzz N, Nasser H, El Shamy A, Abdelnaby R, et al. Bioconjugation as a smart immobilization approach for α- amylase enzyme using stimuli responsive Eudragit-L100 polymer: a robust biocatalyst for applications in pharmaceutical industry. Artif Cells Nanomed Biotechnol. 2019a;47(1):2361–8.
Abdel-Mageed HM, Abuel ezz, N., Radwan, R. Bio-inspired trypsin-chitosan cross-linked enzyme aggregates: a versatile approach for stabilization through carrier-free immobilization. Biotechnologia. 2019b;100:301–9.
Langford A, Bhatnagar B, Walters R, Tchessalov S, Ohtake S. Drying technologies for biopharmaceutical applications: recent developments and future direction. Dry Technol. 2018;36:677–84. https://doi.org/10.1080/07373937.2017.1355318.
Emami F, Vatanara A, Ji Park E, et al. Drying technologies for the stability and bioavailability of biopharmceuticals. Pharmaceutics. 2018;10:131–52. https://doi.org/10.3390/pharmaceutics10030131.
Ziaee A, Albadarin A, Padrela L, et al. Spray drying of pharmaceuticals and biopharmaceuticals: critical parameters and experimental process optimization approaches. Eur J Pharm Sci. 2019;127:300–18.
Ameri M, Maa Y-F. Spray drying of biopharmaceuticals: stability and process considerations. Dry Technol. 2006;24:763–8. https://doi.org/10.1080/03602550600685275.
Elversson J, Millqvist-Fureby A. Particle size and density in spray drying-effects of carbohydrate properties. J Pharm Sci. 2005;94:2049–60.
Arpagaus C, Collenberg A, Rütti D, et al. Nano spray drying for encapsulation of pharmaceuticals. Int J Pharm. 2018;546:194–214. https://doi.org/10.1016/j.ijpharm.2018.05.037.
Costa-Silva A, Nogueira A, Cláudia R. Lipase production by endophytic fungus Cercospora kikuchii: stability of enzymatic activity after spray drying in the presence of carbohydrates. Dry Technol. 2011;29:1112–9. https://doi.org/10.1080/07373937.2011.573153.
Cavalcanti RMF, Martinez MLL, Oliveira WP, Guimarães LHS. Stabilization and application of spray-dried tannase from Aspergillus fumigatus CAS21 in the presence of different carriers. 3 Biotech. 2020;10(4):177. https://doi.org/10.1007/s13205-020-2164-z.
Abdel-Mageed HM, Fouad SA, Teaima MH, et al. Optimization of nano spray drying parameters for production of α-amylase nanopowder for biotheraputic applications using factorial design. Dry Technol. 2019:2152–60. https://doi.org/10.1080/07373937.2019.1565576.
Lipiäinen T, Räikkönen H, Kolu AM, Peltoniemi M, Juppo A. Comparison of melibiose and trehalose as stabilising excipients for spray-dried β-galactosidase formulations. Int J Pharm. 2018;543(1–2):21–8. https://doi.org/10.1016/j.ijpharm.2018.03.035.
Lee G. Rational design of stable protein formulations. In: Carpenter JF, Manning MC, editors. Pharmaceutical biotechnology. Boston: Springer; 2002.
Haggag YA, Faheem AM. Evaluation of nano spray drying as a method for drying and formulation of therapeutic peptides and proteins. Front Pharmacol. 2015;6:140.
Tietz N, Fiereck E. A specific method for serum lipase determination. Clin Chem Acta. 1966;3:352.
Prinn K, Costantino H, Tracy M. Statistical modeling of protein spray drying at the lab scale. AAPS Pharm Sci Tech. 2002;3:1–8.
Arpagaus C. A novel laboratory-scale spray dryer to produce nanoparticles. Dry Technol. 2012;30:1113–21. https://doi.org/10.1080/07373937.2012.686949.
Beck-Broichsitter M, Strehlow B, Kissel T. Direct fractionation of spray-dried polymeric microparticles by inertial impaction. Powder Technol. 2015;286:311–7. https://doi.org/10.1016/j.powtec.2015.08.033.
Ståhl K, Claesson M, Lilliehorn P, Lindén H, Bäckström K. The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int J Pharm. 2002;233:227–37.
Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WL. How sugars protect proteins in the solid state and during drying (review): mechanisms of stabilization in relation to stress conditions. Eur J Pharm Biopharm. 2017;114:288–95.
Raman B, Sundari CS, Balasubramanian D. Amphiphilic properties of polysaccharides: dextrin chains as example. Indian J Biochem Biophys. 1992;29(2):143–7.
Cabral TPF, Bellini NC, Assis KR, Teixeira CCC, Lanchote AD, Cabral H, et al. Microencapsulate Aspergillus niger peptidases from agroindustrial waste wheat bran: spray process evaluation and stability. J Microencapsul. 2017;34(6):560–70. https://doi.org/10.1080/02652048.2017.1367851.
Chan HK, Clark AR, Feeley JC, Kuo MC, Lehrman SR, Pikal-Cleland K, et al. Physical stability of salmon calcitonin spray-dried powders for inhalation. J Pharm Sci. 2004;93(3):792–804. https://doi.org/10.1002/jps.10594.
Grenha A, Seijo B, Remunán-López C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur J Pharm Sci. 2005;25:427–37. https://doi.org/10.1016/j.ejps.2005.04.009.
Sansone F, Esposito T, Mencherini T, Lauro MR, del Gaudio P, Picerno P, et al. Particle technology applied to a lactose/NaCMC blend: production and characterization of a novel and stable spray-dried ingredient. Powder Technol. 2018;329:304–12.
Roos YR, Karel MA. Crystallization of amorphous lactose. J Food Sci. 1992;57:775–7. https://doi.org/10.1111/j.1365-2621.1992.tb08095.x.
Tan S, Ebrahimi A, Liu X, Langrish T. Role of templating agents in the spray drying and postcrystallization of lactose for the production of highly porous powders. Dry Technol. 2018;36(15):1882–91. https://doi.org/10.1080/07373937.2018.1445096.
Schmid K, Arpagaus C, Friess W. Evaluation of the Nano Spray Dryer B-90 for pharmaceutical applications. Pharm Dev Technol. 2010;16(4):287–94.
Landström K, Alsins J, Bergenstahl B. Competitive protein adsorption between bovine serum albumin and b-lactoglobulin during spray-drying. Food Hydrocoll. 2000;14:75–82.
Zhang S, Lei H, Gao X, Xiong X, Wu WD, Wu Z, et al. Fabrication of uniform enzyme-immobilized carbohydrate microparticles with high enzymatic activity and stability via spray drying and spray freeze drying. Powder Technol. 2018;30:40–9. https://doi.org/10.1016/j.powtec.2018.02.02.
Niamnuy C, Poomkokrak L, Dittanet P, Devahastin S. Impacts of spray drying conditions on stability of isoflavones in microencapsulated soybean extract. Dry Technol. 2019;37:1844–62. https://doi.org/10.1080/07373937.2019.1596120.
Jafari S, Masoudi S, Bahrami A. A Taguchi approach production of spray-dried whey powder enriched with nanoencapsulated vitamin D3. Dry Technol. 2019;37:2059–71. https://doi.org/10.1080/07373937.2018.1552598.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Mageed, H.M., Fouad, S.A., Teaima, M.H. et al. Engineering Lipase Enzyme Nano-powder Using Nano Spray Dryer BÜCHI B-90: Experimental and Factorial Design Approach for a Stable Biocatalyst Production. J Pharm Innov 16, 759–771 (2021). https://doi.org/10.1007/s12247-020-09515-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09515-4