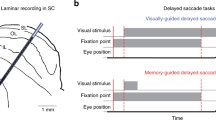
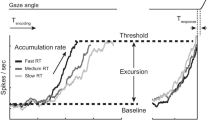
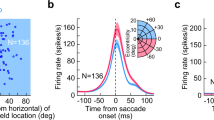
Abstract
Saccades require a spatiotemporal transformation of activity between the intermediate layers of the superior colliculus (iSC) and downstream brainstem burst generator. The dynamic linear ensemble-coding model (Goossens and Van Opstal 2006) proposes that each iSC spike contributes a fixed mini-vector to saccade displacement. Although biologically-plausible, this model assumes cortical areas like the frontal eye fields (FEF) simply provide the saccadic goal to be executed by the iSC and brainstem burst generator. However, the FEF and iSC operate in unison during saccades, and a pathway from the FEF to the brainstem burst generator that bypasses the iSC exists. Here, we investigate the impact of large yet reversible inactivation of the FEF on iSC activity in the context of the model across four saccade tasks. We exploit the overlap of saccade vectors generated when the FEF is inactivated or not, comparing the number of iSC spikes for metrically-matched saccades. We found that the iSC emits fewer spikes for metrically-matched saccades during FEF inactivation. The decrease in spike count is task-dependent, with a greater decrease accompanying more cognitively-demanding saccades. Our results show that FEF integrity influences the readout of iSC activity in a task-dependent manner. We propose that the dynamic linear ensemble-coding model be modified so that FEF inactivation increases the gain of a readout parameter, effectively increasing the influence of a single iSC spike. We speculate that this modification could be instantiated by FEF and iSC pathways to the cerebellum that could modulate the excitability of the brainstem burst generator.







Similar content being viewed by others
References
Anderson, T. J., & MacAskill, M. R. (2013). Eye movements in patients with neurodegenerative disorders. Nature Reviews Neurology. Nature Publishing Group.
Barash, S., Bracewell, R. M., Fogassi, L., Gnadt, J. W., & Andersen, R. A. (1991). Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. Journal of Neurophysiology, 66(3), 1095–1108.
Bruce, C. J., & Goldberg, M. E. (1985). Primate frontal eye fields .I. Single neurons discharging before saccades. Journal of Neurophysiology, 53(3), 603–635.
Crapse, T. B., & Sommer, M. A. (2009). Frontal eye field neurons with spatial representations predicted by their subcortical input. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29(16), 5308–5318.
Dash, S., Peel, T. R., Lomber, S. G., & Corneil, B. D. (2018). Frontal eye field inactivation reduces saccade preparation in the superior Colliculus but does not Alter how preparatory activity relates to saccades of a given latency. eNeuro, 5(2), ENEURO.0024-18.2018.
Deng, S.-Y., Goldberg, M., Segraves, M., Ungerleider, L., & Mishkin, M. (1986). The effect of unilateral ablation of the frontal eye fields on saccadic performance in the monkey. In E.L. Keller, \D.S. Zee, (Eds.), Adaptive processes in the visual and oculomotor systems. (pp. 201–208). Oxford: Pergamon Press.
Dias, E. C., & Segraves, M. A. (1999). Muscimol-induced inactivation of monkey frontal eye field: Effects on visually and memory-guided saccades. Journal of Neurophysiology, 81(5), 2191–2214.
Everling, S., Paré, M., Dorris, M. C., & Munoz, D. P. (1998). Comparison of the discharge characteristics of brain stem Omnipause neurons and superior Colliculus fixation neurons in monkey: Implications for control of fixation and saccade behavior. Journal of Neurophysiology, 79(2), 511–528.
Fuchs, A. F., Robinson, F. R., & Straube, A. (1993). Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. Journal of Neurophysiology, 70(5), 1723–1740.
Gandhi, N. J., & Katnani, H. A. (2011). Motor functions of the superior Colliculus. Annual Review of Neuroscience, 34(1), 205–231.
Gandhi, N. J., & Keller, E. L. (1997). Spatial distribution and discharge characteristics of superior Colliculus neurons Antidromically activated from the Omnipause region in monkey. Journal of Neurophysiology, 78(4), 2221–2225.
Goossens, H. H. L. M., & Van Opstal, A. J. (2000a). Blink-perturbed saccades in monkey. I. Behavioral Analysis. Journal of Neurophysiology, 83(6), 3411–3429.
Goossens, H. H. L. M., & Van Opstal, A. J. (2000b). Blink-perturbed saccades in monkey. . II. Superior Colliculus Activity. Journal of Neurophysiology, 83(6), 3430–3452.
Goossens, H. H. L. M., & Van Opstal, A. J. (2006). Dynamic ensemble coding of saccades in the monkey superior Colliculus. Journal of Neurophysiology, 95(4), 2326–2341.
Goossens, H. H. L. M., & Van Opstal, A. J. (2012). Optimal control of saccades by spatial-temporal activity patterns in the monkey superior Colliculus. PLoS Computational Biology, 8(5), e1002508.
Groh, J. M. (2001). Converting neural signals from place codes to rate codes. Biological Cybernetics, 85(3), 159–165.
Hafed, Z. M., & Chen, C.-Y. (2016). Sharper, stronger, faster upper visual field representation in primate superior Colliculus. Current Biology, 26(13), 1647–1658.
Hanes, D. P., & Wurtz, R. H. (2001). Interaction of the frontal eye field and superior colliculus for saccade generation. Journal of Neurophysiology, 85(2), 804–815.
Hikosaka, O., Takikawa, Y., & Kawagoe, R. (2000). Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews, 80(3), 953–978.
Huerta, M. F., Krubitzer, L. A., & Kaas, J. H. (1986). Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: I. subcortical connections. The Journal of Comparative Neurology, 253(4), 415–439.
Kasap, B., & Van Opstal, A. J. (2017). A spiking neural network model of the midbrain superior colliculus that generates saccadic motor commands. Biological Cybernetics, 111(3–4), 249–268.
Kasap, B., & Van Opstal, A. J. (2019). Microstimulation in a spiking neural network model of the midbrain superior colliculus. PLoS Computational Biology, 15(4), e1006522.
Keating, E. G., & Gooley, S. G. (1988). Saccadic disorders caused by cooling the superior colliculus or the frontal eye field, or from combined lesions of both structures. Brain Research, 438(1–2), 247–255.
Lomber, S. G., Payne, B. R., & Horel, J. A. (1999). The cryoloop: An adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. Journal of Neuroscience Methods, 86(2), 179–194.
McPeek, R. M., & Keller, E. L. (2002). Saccade target selection in the superior colliculus during a visual search task. Journal of Neurophysiology, 88(4), 2019–2034.
Miura, K., & Optican, L. M. (2006). Membrane channel properties of premotor excitatory burst neurons may underlie saccade slowing after lesions of omnipause neurons. Journal of Computational Neuroscience, 20(1), 25–41.
Moschovakis, A. K., Kitama, T., Dalezios, Y., Petit, J., Brandi, A. M., & Grantyn, A. A. (1998). An anatomical substrate for the spatiotemporal transformation. The Journal of neuroscience : the official journal of the Society for Neuroscience, 18(23), 10219–10229.
Munoz, D. P., & Wurtz, R. H. (1995). Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. Journal of Neurophysiology, 73(6), 2313–2333.
Nichols, M. J., & Sparks, D. L. (1996). Component stretching during oblique stimulation-evoked saccades: The role of the superior colliculus. Journal of Neurophysiology, 76(1), 582–600.
Ottes, F. P., Van Gisbergen, J. A. M., & Eggermont, J. J. (1986). Visuomotor fields of the superior colliculus: A quantitative model. Vision Research, 26(6), 857–873.
Peel, T. R., Dash, S., Lomber, S. G., & Corneil, B. D. (2017). Frontal eye field inactivation diminishes superior Colliculus activity, but delayed saccadic accumulation governs reaction time increases. The Journal of neuroscience : the official journal of the Society for Neuroscience, 37(48), 11715–11730.
Peel, T. R., Hafed, Z. M., Dash, S., Lomber, S. G., & Corneil, B. D. (2016). A causal role for the cortical frontal eye fields in microsaccade deployment. PLoS Biology, 14(8), e1002531.
Peel, T. R., Johnston, K., Lomber, S. G., & Corneil, B. D. (2014). Bilateral saccadic deficits following large and reversible inactivation of unilateral frontal eye field. Journal of Neurophysiology, 111(2), 415–433.
Quaia, C., Lefèvre, P., & Optican, L. M. (1999). Model of the control of saccades by superior colliculus and cerebellum. Journal of Neurophysiology, 82(2), 999–1018.
Raybourn, M. S., & Keller, E. L. (1977). Colliculoreticular organization in primate oculomotor system. Journal of Neurophysiology, 40(4), 861–878.
Robinson, D. A. (1972). Eye movements evoked by collicular stimulation in the alert monkey. Vision Research, 12(11), 1795–1808.
Sato, H., & Noda, H. (1992). Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis. Experimental Brain Research, 88(2), 455–458.
Schiller, P. H., True, S. D., & Conway, J. L. (1980). Deficits in eye movements following frontal eye-field and superior colliculus ablations. Journal of Neurophysiology, 44(6), 1175–1189.
Scudder, C. A., Kaneko, C. R., & Fuchs, A. F. (2002). The brainstem burst generator for saccadic eye movements: A modern synthesis. Experimental Brain Research, 142(4), 439–462.
Segraves, M. A. (1992). Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. Journal of Neurophysiology, 68(6), 1967–1985.
Smalianchuk, I., Jagadisan, U. K., & Gandhi, N. J. (2018). Instantaneous midbrain control of saccade velocity. Journal of Neuroscience, 38(47), 10156–10167.
Soetedjo, R., Kaneko, C. R. S., & Fuchs, A. F. (2002). Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. Journal of Neurophysiology, 87(2), 679–695.
Sommer, M. A., & Tehovnik, E. J. (1997). Reversible inactivation of macaque frontal eye field. Experimental Brain Research, 116(2), 229–249.
Sparks, D. L. (1986). Translation of sensory signals into commands for control of saccacid eye movements: Role of primate superior colliculus. Physiological Reviews, 66, 118–171.
Sparks, D. L., & Mays, L. E. (1990). Signal transformations required for the generation of saccadic eye movements. Annual Review of Neuroscience, 13(1), 309–336.
Sparks, D. L. (2002). The brainstem control of saccadic eye movements. Nature Reviews Neuroscience, 3(12), 952–964.
Thompson, K. G., Hanes, D. P., Bichot, N. P., & Schall, J. D. (1996). Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. Journal of Neurophysiology, 76(6), 4040–4055.
Van der Willigen, R. F., Goossens, H. H. L. M., & Van Opstal, A. J. (2011). Linear visuomotor transformations in midbrain superior colliculus control saccadic eye movements. Journal of Integrative Neuroscience, 10(03), 277–301.
Van Opstal, A. J., & Goossens, H. H. L. M. (2008). Linear ensemble-coding in midbrain superior colliculus specifies the saccade kinematics. Biological Cybernetics, 98(6), 561–577.
Van Opstal, A. J., & Van Gisbergen, J. A. M. (1989). A nonlinear model for collicular spatial interactions underlying the metrical properties of electrically elicited saccades. Biological Cybernetics, 60(3), 171–183.
White, B. J., & Munoz, D. P. (2011). The superior colliculus. In S. P. Liversedge, I. Gilchrist, & S. Everling (Eds.), The Oxford Handbook of Eye Movements (pp. 195–214). Oxford University Press.
Wurtz, R. H., Sommer, M. A., Paré, M., & Ferraina, S. (2001). Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Research, 41(25–26), 3399–3412.
Xiong, G., Hiramatsu, T., & Nagao, S. (2002). Corticopontocerebellar pathway from the prearcuate region to hemispheric lobule VII of the cerebellum: An anterograde and retrograde tracing study in the monkey. Neuroscience Letters, 322(3), 173–176.
Yoshida, K., Iwamoto, Y., Chimoto, S., & Shimazu, H. (1999). Saccade-related inhibitory input to pontine omnipause neurons: An intracellular study in alert cats. Journal of Neurophysiology, 82(3), 1198–1208.
Acknowledgements
This work was supported by operating grants from the Canadian Institutes of Health Research to BDC (MOPs: 93796, 123247 and 142317) and the Natural Sciences and Engineering Research Council (NSERC; RGPIN-311680). TRP was supported by an Ontario Graduate Scholarship and TRP and SD were supported by funding from an NSERC CREATE grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Action Editor: Aasef G. Shaikh
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Vision and Action
Guest Editors: Aasef Shaikh and Jeffrey Shall
Significance statement
One of the enduring puzzles in the oculomotor system is how it achieves the spatiotemporal transformation, converting spatial activity within the intermediate layers of the superior colliculus (iSC) into a rate code within the brainstem burst generator. The spatiotemporal transformation has traditionally been viewed as the purview of the oculomotor brainstem. Here, within the context of testing a biologically-plausible model of the spatiotemporal transformation, we show that reversible inactivation of the frontal eye fields (FEF) decreases the number of spikes issued by the iSC for metrically-matched saccades, with greater decreases accompanying more cognitively-demanding tasks. These results show that signals from the FEF influence the spatiotemporal transformation.
Rights and permissions
About this article
Cite this article
Peel, T.R., Dash, S., Lomber, S.G. et al. Frontal eye field inactivation alters the readout of superior colliculus activity for saccade generation in a task-dependent manner. J Comput Neurosci 49, 229–249 (2021). https://doi.org/10.1007/s10827-020-00760-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-020-00760-7