Abstract
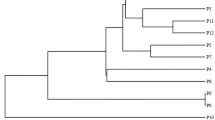
Weed resistance to herbicides is a serious agricultural issue that threatens the sustainability of world food production, as its widespread appearance has reduced the diversity of weed control practices and posed economic and environmental risks. Wild mustard (Sinapis arvensis), a very competitive weed in winter cereals, has evolved resistance to ALS-inhibiting herbicides used in Greece. As growth rate and genetic structure of weeds affect their competitive ability against crops, the purpose of this work was to study the effect of geographical origin and resistance profile on growth rate and genetic structure of five S. arvensis populations. In particular, the cross-resistant to tribenuron and imazamox P2, P3 and P4 populations originating from the counties of Larissa, Phthiotida, and Pieria, respectively, the resistant to tribenuron P1 originating from Chalkidiki and the susceptible PS originating from Thessaloniki were evaluated. The growth rate was studied by determining fresh weight, number of leaves, height, and seed weight of plants, whereas the genetic structure by using six inter simple sequence repeat (ISSR) molecular markers. Regarding growth rate, the b slope values of the linear equation fitted on fresh weight regression against sampling time were 10.87, 9.94 and 11.41 for P2, P3 and P4 populations, respectively, which were higher than those of P1 (8.03) and PS (6.79) populations, while the b slopes of leaf number were 3.8 and 4.63 for P3 and P1 populations, which were higher than those of PS (2.1), P2 (2.79) and P4 (2.84). The use of ISSR markers showed also differences between populations in their genetic structure parameters [genetic diversity (h), Shannon index (I), number of alleles (Na), percentage of polymorphic loci for each population (P%), variance among and within populations, and FST value, that is a measure of differentiation among populations due to genetic structure], which attributed to origin and herbicide resistance profile. The ISSR bands indicated that 80% of the molecular variation was within populations and 20% among populations. In addition, the dendrogram produced by the combination of GenALEx and MEGA7 statistical software, along with the corresponding PCoA graphical output indicated similar groups of the individuals belonging in these populations.



Similar content being viewed by others
References
Almeida-Pereira CS, Silva AVC, Alves RP, Feitosa-Alcantara RB, Arrigoni-Blank1 MF, Alvares-Carvalho SV, Costa TS, White LAS, Pinto VS, Sampaio TS, Blank AF (2017) Genetic diversity of native populations of Croton tetradenius Baill. using ISSR markers. Genet Mol Res 16(2). https://doi.org/10.4238/gmr16029602
Altop EK, Jabran K, Mennan H (2017) Determination of morphological and genetic diversity of ALS (Acetolactate Synthase)-herbicide-resistant Echinochloa oryzoides biotypes in rice. Int J Agric Biol 20:628–636
Anthimidou E, Ntoanidou S, Madesis P, Eleftherohorinos I (2020) Mechanisms of Lolium rigidum multiple resistance to ALS- and ACCase-inhibiting herbicides and their impact on plant fitness. Pestic Biochem Physiol 164:65–72
Baucom RS (2019) Evolutionary and ecological insights from herbicide-resistant weeds: what have we learned about plant adaptation, and what is left to uncover? New Phytol 223:68–82
Boutsalis P, Karotam J, Powles SB (1999) Molecular basis of resistance to acetolactate synthase-inhibiting herbicides in Sisymbrium orientale and Brassica tournefortii. Pestic Sci 55:507–516
Christoffers MJ, Nandula VK, Howatt KA, Wehking TR (2006) Target-site resistance to acetolactate synthase inhibitors in wild mustard (Sinapis arvensis). Weed Sci 54:191–197
Claerhout S, Reheul D, De Cauwer B (2015) Sensitivity of Echinochloa crus-galli populations to maize herbicides: a comparison between cropping systems. Weed Res 55:470–481
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bulletin 19:11–15
Délye C, Jasieniuk M, Le Corre V (2013) Deciphering the evolution of herbicide resistance in weeds. Trends Genet 29:649–658
Eberlein CV, Guttieri MJ, Mallory-Smith CA, Thill DC, Baerg RJ (1997) Altered acetolactate synthase activity in ALS-inhibitor resistant prickly lettuce (Lactuca serriola). Weed Sci 45:212–217
Ganopoulos I, Kalivas A, Kavroulakis N, Xanthopoulou A, Mastrogianni A, Koubouris G, Madesis P (2015) Genetic diversity of Barbary fig (Opuntia ficus-indica) collection in Greece with ISSR molecular markers. Plant Gene 2:29–33
Gherekhloo J, Hatami ZM, Alcántara-de la Cruz R, Sadeghipour HR, De Prado R (2018) Continuous use of tribenuron-methyl selected for cross-resistance to acetolactate synthase inhibiting herbicides in wild mustard (Sinapis arvensis). Weed Sci 66:424–432
Heap I (2020) International survey of herbicide resistant weeds. http://www.weedscience.org. Accessed 20 Mar 2020
Houmanat K, Charafi J, Mazouz H, El Fechtali M, Nabloussi A (2016) Genetic diversity analysis of safflower (Carthamus tinctorius) accessions from different geographic origins using ISSR markers. Int J Agric Biol 18:1081–1087
Huangfu C-h, Song X-L, Qiang S (2009) ISSR variation within and among wild Brassica juncea populations: implication for herbicide resistance evolution. Genet Resour Crop Evol 56:913–924
Kaloumenos NS, Tsioni VC, Daliani EG, Papavassileiou SE, Vassileiou AG, Lautidou PN, Eleftherohorinos IG (2012) Multiple Pro-197 substitutions in the acetolactate synthase of rigid ryegrass (Lolium rigidum) and their impact on chlorsulfuron activity and plant growth. Crop Prot 38:35–43
Karn E, Jasieniuk M (2017) Genetic diversity and structure of Lolium perenne ssp. multiflorum in California vineyards and orchards indicates potential for spread of herbicide resistance via gene flow. https://doi.org/10.1111/eva.12478
Khaledi R, Fayaz F, Kahrizi D, Talebi R (2019) PCR-based identifcation of point mutation mediating acetolactate synthase-inhibiting herbicide resistance in weed wild mustard (Sinapis arvensis). Mol Biol Rep. https://doi.org/10.1007/s11033-019-04967-5
Lewontin RC (1972) The apportionment of human diversity. Evol Biol 6:381–398
Li M, Yu Q, Han H, Vila-Aiub M, Powles SB (2013) ALS herbicide resistance mutations in Raphanus raphanistrum: evaluation of pleiotropic effects on vegetative growth and ALS activity. Pest Manag Sci 69:689–695
Linhart YB, Grant MC (1996) Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Evol Syst 27:237–277
Liu W, Bai S, Jia S, Guo W, Zhang L, Li W, Wang J (2017) Comparison of ALS functionality and plant growth in ALS-inhibitor susceptible and resistant Myosoton aquaticum L. Pestic Biochem Physiol 142:111–119
Luo C, He X, Chen H, Ou S, Gao M, Brown JS, Tondo CT, Schnell RJ (2011) Genetic diversity of mango cultivars estimated using SCoT and ISSR markers. Biochem Syst Ecol 39:676–684
Menegat A, Bailly GC, Aponte R, Heinrich GMT, Sievernich B, Gerhards R (2016) Acetohydroxyacid synthase (AHAS) amino acid substitution Asp376Glu in Lolium perenne: effect on herbicide efficacy and plant growth. J Plant Dis Prot 123:145–153
Mengistu LW, Messersmith CG (2002) Genetic diversity of kochia. Weed Sci 50:498–503
Mengistu LW, Messersmith CG, Christoffer MJ (2005) Genetic diversity of herbicide-resistant and—susceptible Avena fatua populations in North Dakota and Minnesota. Weed Res 45:413–423
Mohammadi SA, Prasanna BM (2003) Analysis of genetic diversity in crop plants—salient statistical tools and considerations. Crop Sci 43:1235–1248
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Neirmans PG, Hedrick PW (2011) Assessing population structure: FST and related measures. Mol Ecol Resour 11:5–18
Ntoanidou S, Madesis P, Diamantidis G, Eleftherohorinos I (2017) Trp574 substitution in the acetolactate synthase of Sinapis arvensis confers cross-resistance to tribenuron and imazamox. Pestic Biochem Physiol 142:9–14
Ntoanidou S (2019) Study of susceptible and resistant populations of Sinapis arvensis and Rapistrum rugosum to ALS-inhibiting herbicides. PhD thesis, Aristotle University of Thessaloniki, p 149.
Papapanagiotou AP, Paresidou MI, Kaloumenos NS, Eleftherohorinos IG (2015) ACCase mutations in Avena sterilis populations and their impact on plant fitness. Pestic Biochem Physiol 123:40–48
Peakall R, Smouse PE (2005) GENALEX 6: genetic analysis in excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pratsinakis ED, Ntoanidou S, Polidoros A, Dordas C, Madesis P, Eleftherohorinos I, Menexes G (2020) Comparison of hierarchical clustering methods for binary data from molecular markers. Int J Data Anal Techn Strat 12(3):190
Preston C, Stone LM, Rieger MA, Baker J (2006) Multiple effects of a naturally occurring proline to threonine substitution within acetolactate synthase in two herbicide-resistant populations of Lactuca serriola. Pestic Biochem Physiol 84:227–235
Safahani Langeroudi AR, Kamkar B (2009) Field screening of canola (Brassica napus) cultivars against wild mustard (Sinapis arvensis) using competition indices and some empirical yield loss models in Golestan Province, Iran. Crop Prot 28:577–582
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan MK, Medd RW (2002) Characterisation of the acetolactate synthase (ALS) gene of Raphanus raphanistrum L. and the molecular assay of mutations associated with herbicide resistance. Plant Sci 163:195–200
Tardif FJ, Rajcan I, Costea M (2006) A mutation in the herbicide target site acetohydroxyacid synthase produces morphological and structural alterations and reduces fitness in Amaranthus powellii. New Phytol 169:251–264
Warwick SI, Beckie HJ, Thomas AG, McDonald T (2000) The biology of Canadian weeds. 8. Sinapis arvensis L. (updated). Can J Plant Sci 55:171–183
Warwick SI, Sauder C, Beckie HJ (2005) Resistance in Canadian biotypes of wild mustard (Sinapis arvensis) to acetolactate synthase inhibiting herbicides. Weed Sci 53:631–639
Yanniccari M, Vila-Aiub M, Istilart C, Acciaresi H, Castro AM (2016) Glyphosate resistance in perennial ryegrass (Lolium perenne L.) is associated with a fitness penalty. Weed Sci 64:71–79
Yu Q, Han H, Vila-Aiub MM, Powles SB (2010) AHAS herbicide resistance endowing mutations: effect on AHAS functionality and plant growth. J Exp Bot 61:3925–3934
Yu Q, Powles SB (2014) Resistance to AHAS inhibitor herbicides: current understanding. Pest Manag Sci 70:1340–1350
Zafar-Pashanezhada M, Shahbazia E, Golkarb P, Shiran B (In press) Genetic variation of Eruca sativa L. genotypes revealed by agromorphological traits and ISSR molecular markers. Ind Crop Prod. https://doi.org/10.1016/j.indcrop.2019.111992
Zangeneh HS, Chamanabad HRM, Zand E, Asghari A, Alamisaeid K, Travlos IS, Alebrahim MT (2016) Study of fitness cost in three rigid ryegrass populations susceptible and resistant to acetyl-CoA carboxylase inhibiting herbicides. Front Ecol Evol 4:142
Zhivotovsky LA (1999) Estimating population structure in diploids with multilocus dominant DNA markers. Mol Ecol 8:907–913
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)—anchored polymerase chain reaction amplification. Genomics 20:176–183
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Ntoanidou, S., Madesis, P., Menexes, G. et al. Growth rate and genetic structure of Sinapis arvensis susceptible and herbicide resistant populations originating from Greece. Euphytica 216, 185 (2020). https://doi.org/10.1007/s10681-020-02723-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02723-6