Abstract
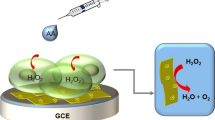
The development of metal nanoparticles (MNP) combined with a metal-organic framework (MOF) has received more and more attention due to its excellent synergistic catalytic ability, which can effectively broaden the scope of catalytic reactions and enhance the catalytic ability. In this work, we developed a novel ternary nanocomposite named Cu2O-mediated Au nanoparticle (Au NP) grown on MIL-53(Fe) for real-time monitoring of hydrogen peroxide (H2O2) released from living cells. First, Cu2O-MIL-53(Fe) was prepared by redox assembly technology, which provided the growth template, and active sites for AuCl4−. Au@Cu2O-MIL-53(Fe)/GCE biosensor was prepared by further loading nano-Au uniformly on the surface of Cu2O by electrochemical deposition. Compared to individual components, the hybrid nanocomposite showed superior electrochemical properties as electrode materials due to the synergistic effect between AuNPs, Cu2O, and MIL-53(Fe). Electrochemical measurement showed that the Au@Cu2O-MIL-53(Fe)/GCE biosensor presented a satisfactory catalytic activity towards H2O2 with a low detection limit of 1.01 μM and sensitivity of 351.57 μA mM−1 cm−2 in the linear range of 10–1520 μM. Furthermore, this biosensor was successfully used for the real-time monitoring of dynamic H2O2 activated by PMA released from living cells. And the great results of confocal fluorescence microscopy of the co-culture cells with PMA and Au@Cu2O-MIL-53(Fe) verified the reliability of the biosensor, suggesting its potential application to the monitoring of critical pathological processes at the cellular level.









Similar content being viewed by others
References
Dong Z, Yang Zj, Hao Y, Feng L. Fabrication of H2O2-driven nanoreactors for innovative cancer treatments. Nanoscale. 2019;11:16164–86.
Lim CK, Lee YD, Na J, Oh JM, Song H, Kim K, et al. Chemiluminescence-generating nanoreactor formulation for near-infrared imaging of hydrogen peroxide and glucose level in vivo. Adv Funct Mater. 2010;20(16):2644–8.
Qiao J, Liu Z, Tian Y, Wu M, Niu Z. Multifunctional self-assembled polymeric nanoprobes for FRET-based ratiometric detection of mitochondrial H2O2 in living cells. Chem Commun. 2015;51(17):3641–4.
Zhou H, Liu J, Zhang S. Quantum dot-based photoelectric conversion for biosensing applications. TrAC Trends Anal Chem. 2015;67:56–73.
Abbas K, Hardy M, Poulhès F, Karoui H, Tordo P, Ouari O, et al. Detection of superoxide production in stimulated and unstimulated living cells using new cyclic nitrone spin traps. Free Radic Biol Med. 2014;71:281–90.
Chen W, Cai S, Ren Q-Q, Wen W, Zhao Y-D. Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst. 2012;137(1):49–58.
Meng L, Jin J, Yang G, Lu T, Zhang H, Cai C. Nonenzymatic electrochemical detection of glucose based on palladium-single-walled carbon nanotube hybrid nanostructures. Anal Chem. 2009;81(17):7271–80.
Li M, Wu J, Su H, Tu Y, Shang Y, He Y, et al. Ionic liquid-polypyrrole-gold composites as enhanced enzyme immobilization platforms for hydrogen peroxide sensing. Sensors. 2019;19(3):6402.
Kong Y-T, Boopathi M, Shim Y-B. Direct electrochemistry of horseradish peroxidase bonded on a conducting polymer modified glassy carbon electrode. Biosens Bioelectron. 2003;19(3):227–32.
Li L, Wang X, Liu G, Wang Z, Wang F, Guo X, et al. Reproducible preparation of a stable polypyrrole-coated-silver nanoparticles decorated polypyrrole-coated-polycaprolactone-nanofiber-based cloth electrode for electrochemical sensor application. Nanotechnology. 2015;26(44):445704.
Zhang W, Wang C, Guan L, Peng M, Li K, Lin Y. A non-enzymatic electrochemical biosensor based on Au@PBA(Ni-Fe):MoS nanocubes for stable and sensitive detection of hydrogen peroxide released from living cells. J Mater Chem B. 2019;7(48):7704–12.
Zhao W, Jin J, Wu H, Wang S, Fneg C, Yang S, et al. Electrochemical hydrogen peroxide sensor based on carbon supported Cu@Pt core-shell nanoparticles. Mater Sci Eng C Mater Biol Appl. 2017;78:185–90.
Li C, Chen D, Wang Y, Lai X, Peng J, Wang X, et al. Simultaneous electrochemical detection of nitrite and hydrogen peroxide based on 3D Au-rGO/FTO obtained through a one-step synthesis. Sensors. 2019;19(6):1304.
Sun Y, Luo M, Meng X, Xiang J, Wang L, Ren Q, et al. Graphene/intermetallic PtPb nanoplates composites for boosting electrochemical detection of H2O2 released from cells. Anal Chem. 2017;89(6):3761–7.
Bai J, Jiang X. A facile one-pot synthesis of copper sulfide-decorated reduced graphene oxide composites for enhanced detecting of H2O2 in biological environments. Anal Chem. 2013;85(17):8095–101.
Du X, Chen Y, Dong W, Han B, Liu M, Chen Q, et al. A nanocomposite-based electrochemical sensor for non-enzymatic detection of hydrogen peroxide. Oncotarget. 2017;8(8):13039–47.
Zhou Y, Li C, Hao Y, Ye B, Xu M. Oriented growth of cross-linked metal-organic framework film on graphene surface for non-enzymatic electrochemical sensor of hydrogen peroxide in disinfectant. Talanta. 2018;188:282–7.
Wang C, Zhou M, Ma Y, Tan H, Wang Y, Li Y. Hybridized polyoxometalate-based metal-organic framework with Ketjenblack for the nonenzymatic detection of H2O2. Chem Asian J. 2018;13(16):2054–9.
Ling W, Hao Y, Fau-Wang H, Wang H, Fau-Xu H, Xu H, et al. A novel Cu-metal-organic framework with two-dimensional layered topology for electrochemical detection using flexible sensors. Nanotechnology. 2019;30(42):424002.
He G, Gao F, Li W, Li P, Zhang X, Yin H, et al. Electrochemical sensing of H2O2 released from living cells based on AuPd alloy-modified PDA nanotubes. Anal Methods. 2019;11(12):1651–6.
Zhu L, Zhang Y, Xu P, Wen W, Li X, Xu J. PtW/MoS2 hybrid nanocomposite for electrochemical sensing of H2O2 released from living cells. Biosens Bioelectron. 2016;80:601–6.
Ramaraj S, Sakthivel M, Chen SM, Lou BS, Ho KC. Defect and additional active sites on the basal plane of manganese-doped molybdenum diselenide for effective enzyme immobilization: in vitro and in vivo real-time analyses of hydrogen peroxide sensing. ACS Appl Mater Interfaces. 2019;11(8):7862–71.
Qiu W, Zhu Q, Gao F, Gao F, Huang J, Pan Y, et al. Graphene oxide directed in-situ synthesis of Prussian blue for non-enzymatic sensing of hydrogen peroxide released from macrophages. Mater Sci Eng C Mater Biol Appl. 2017;72:692–700.
Dai H, Chen Y, Niu X, Pan C, Chen H, Chen X. High-performance electrochemical biosensor for nonenzymatic H2O2 sensing based on Au@C-Co3O4 heterostructures. Biosens Bioelectron. 2018;118:36–43.
Yaghi OM, O'Keeffe M, Ocking NW, Chae HK, Eddaoudi M, et al. Reticular synthesis and the design of new marterials. Nature. 2003;423:705–14.
Fu Y, Dai J, Ge Y, Zhang Y, Ke H, Zhang W. A novel non-enzymatic electrochemical hydrogen peroxide sensor based on a metal-organic framework/carbon nanofiber composite. Molecules. 2018;23(10):2552.
Feng J, Wang H, Ma Z. Ultrasensitive amperometric immunosensor for the prostate specific antigen by exploiting a Fenton reaction induced by a metal-organic framework nanocomposite of type Au/Fe-MOF with peroxidase mimicking activity. Mikrochim Acta. 2020;187(1):95.
Ling P, Qian C, Yu J, Gao F. Artificial nanozyme based on platinum nanoparticles anchored metal-organic frameworks with enhanced electrocatalytic activity for detection of telomeres activity. Biosens Bioelectron. 2020;149:111838.
Chen C, Xiong D, Gu M, Lu C, Yi F-Y, Ma X. MOF-derived bimetallic CoFe-PBA composites as highly selective and sensitive electrochemical sensors for hydrogen peroxide and nonenzymatic glucose in human serum. ACS Appl Mater Interfaces. 2020;12(31):35365–74.
Horike S, Dinca M, Tamaki K. Long JRJJotACS. Size-selective Lewis acid catalysis in a microporous metal-organic framework with exposed Mn2+ coordination sites. J Am Chem Soc. 2008;130(18):5854–5.
Devic T, Horcajada P, Serre C, et al. Functionalization in flexible porous solids: effects on the pore opening and the host-guest interactions. J Am Chem Soc. 2010;132(3):1127–36.
Ma J, Bai W, Zheng J. Non-enzymatic electrochemical hydrogen peroxide sensing using a nanocomposite prepared from silver nanoparticles and copper (II)-porphyrin derived metal-organic framework nanosheets. Mikrochim Acta. 2019;186(7):482.
Pan Y, Yuan B, Li Y, He D. Multifunctional catalysis by Pd@MIL-101: one-step synthesis of methyl isobutyl ketone over palladium nanoparticles deposited on a metal-organic framework. Chem Commun (Camb). 2010;46(13):2280–2.
Huang Y, Lin Z, Cao R. Palladium nanoparticles encapsulated in a metal-organic framework as efficient heterogeneous catalysts for direct C2 arylation of indoles. Chemistry. 2011;17(45):12706–12.
Zhao M, Deng K, He L, Liu Y, Li G, Zhao H, et al. Core-shell palladium nanoparticle@metal-organic frameworks as multifunctional catalysts for cascade reactions. J Am Chem Soc. 2014;136(5):1738–41.
Szécsényi Á, Li G, Gascon J, Pidko EA. Unraveling reaction networks behind the catalytic oxidation of methane with H2O2 over a mixed-metal MIL-53(Al,Fe) MOF catalyst. Chem Sci. 2018;9(33):6765–73.
Li H, Ban L, Niu Z, Huang X, Meng P, Han X, et al. Application of CuxO-FeyOz nanocatalysts in ethynylation of formaldehyde. Nanomaterials (Basel). 2019;9(9):1301.
Liang Y, Chen Z, Yao W, Wang P, Yu S, Wang X. Decorating of Ag and CuO on cu nanoparticles for enhanced high catalytic activity to the degradation of organic pollutants. Langmuir. 2017;33(31):7606–14.
Zhu XD, Wang KX, Yan DJ, Le SR, Ma RJ, Sun KN, et al. Creating a synergistic interplay between tubular MoS2 and particulate Fe3O4 for improved lithium storage. Chem Commun (Camb). 2015;51(59):11888–91.
Liang R, Jing F, Shen L, Qin N. Wu LJJoHM. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J Hazard Mater. 2015;287C:364–72.
Wang C, Zhang Y, Li Y, Liu J, Wu QH, Jiang J, et al. Synthesis of fluorine-doped α-Fe2O3 nanorods toward enhanced lithium storage capability. Nanotechnology. 2017;28(6):065401.
Biesinger MC, Payne BP, Grosvenor AP, Lau LWM, Gerson AR, Smart RSC. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci. 257(7):2717–30.
Luo J, Liu Y, Niu Y, Jiang Q, Huang R, Zhang B, et al. Insight into the chemical adsorption properties of CO molecules supported on Au or Cu and hybridized Au-CuO nanoparticles. Nanoscale. 2017;9(39):15033–43.
Bulushev DA, Yuranov I, Suvorova EI, Buffat PA, Kiwi-Minsker L. Highly dispersed gold on activated carbon fibers for low-temperature CO oxidation. J Catal. 2004;224(1):8–17.
Yang M, Allard LF. Flytzani-Stephanopoulos MJJotACS. Atomically dispersed Au-(OH) x species bound on titania catalyze the low-temperature water-gas shift reaction. J Am Chem Soc. 2013;135(10):3768–71.
Yang Q, Jiang H-L. Oxidation or reduction state of Au stabilized by an MOF: active site identification for the three-component coupling reaction. Small Methods. 2018;2:1800216.
Yang P, Pan J, Liu Y, Zhang X, Feng J, Hong S, et al. Insight into the role of unsaturated coordination O2c-Ti5c-O2c sites on selective glycerol oxidation over AuPt/TiO2 catalysts. ACS Catal. 2018;9(1):188–99.
Daemi S, Ghasemi S, Akbar Ashkarran A. Electrospun CuO-ZnO nanohybrid: tuning the nanostructure for improved amperometric detection of hydrogen peroxide as a non-enzymatic sensor. J Colloid Interface Sci. 2019;550:180–9.
Dang W, Sun Y, Jiao H, Xu L, Lin M. AuNPs-NH2/cu-MOF modified glassy carbon electrode as enzyme-free electrochemical sensor detecting H2O2. J Electroanal Chem. 2020;856:113592.
Li D, Meng L, Dang S, Jiang D, Shi W. Hydrogen peroxide sensing using Cu2O nanocubes decorated by Ag-Au alloy nanoparticles. J Alloys Compd. 2017;690:1–7.
Li D, Meng L, Xiao P, Jiang D, Dang S, Chen M. Enhanced non-enzymatic electrochemical sensing of hydrogen peroxide based on Cu2O nanocubes/Ag-Au alloy nanoparticles by incorporation of RGO nanosheets. J Electroanal Chem. 2017;791:23–8.
Doğan HÖ, Çepni E, Urhan BK, Eryiğit M. Non-enzymatic amperometric detection of H2O2 on one-step electrochemical fabricated Cu2O/electrochemically reduced graphene oxide nanocomposite. Chem Select. 2019;4(28):8317–21.
Cheng C, Zhang C, Gao X, Zhuang Z, Du C, Chen W. 3D network and 2D paper of reduced graphene oxide/CuO composite for electrochemical sensing of hydrogen peroxide. Anal Chem. 2018;90(3):1983–91.
Zhou L, Kuai L, Li W, Geng B. Ion-exchange route to Au-Cu(x)OS yolk-shell nanostructures with porous shells and their ultrasensitive H2O2 detection. ACS Appl Mater Interfaces. 2012;4(12):6463–7.
Dong W, Ren Y, Zhang Y, Chen Y, Zhang C, Bai Z, et al. Synthesis of Pb nanowires-au nanoparticles nanostructure decorated with reduced graphene oxide for electrochemical sensing. Talanta. 2017;165:604–11.
Ngamaroonchote A, Sanguansap Y, Wutikhun T, Karn-Orachai K. Highly branched gold-copper nanostructures for non-enzymatic specific detection of glucose and hydrogen peroxide. Mikrochim Acta. 2020;187(10):559.
Liu H, Chen Q, Cheng X, Wang Y, Zhang Y, Fan G. Sustainable and scalable in-situ fabrication of Au nanoparticles and Fe3O4 hybrids as highly efficient electrocatalysts for the enzyme-free sensing of H2O2 in neutral and basic solutions. Sensors Actuators B Chem. 2020;314:128067.
Heydaryan K, Kashi M, Sharifi N, Ranjbar-Azad M. Efficiency improvement in non-enzymatic H2O2 detection induced by the simultaneous synthesis of Au and Ag nanoparticles in an RGO/Au/Fe3O4/Ag nanocomposite. New J Chem. 2020;44(21):9037–45.
Takatoshi T, Hitomi O, Katsuyuki M, Ryokei O, Chiyoko I. Phorbol 12-myristate 13-acetate (PMA)-induced oxyradical production in rheumatoid synovial cells. Jpn J Pharmacol. 1997;73:347–51.
Zhao Y, Huo D, Bao J, Yang M, Chen M, Hou J, et al. Biosensor based on 3D graphene-supported Fe3O4 quantum dots as biomimetic enzyme for in situ detection of H2O2 released from living cells. Sensors Actuators B Chem. 2017;244:1037–44.
Wang Q, Yang Y, Gao F, Ni J, Zhang Y, Lin Z. Graphene oxide directed one-step synthesis of flowerlike graphene@HKUST-1 for enzyme-free detection of hydrogen peroxide in biological samples. ACS Appl Mater Interfaces. 2016;8(47):32477–87.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 81772290), Graduate Scientific Research and Innovation Foundation of Chongqing, China (Grant No.CYB20070, CYS2007), Chongqing science and technology commission (CSTC2018jcyjAX0062), Chongqing Graduate Tutor Team Construction Project, Analytical and Testing Center of Chongqing University for (SEM/TEM/the characterization of EDS/XRD/XPS), and the sharing fund of Chongqing University’s large equipment.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 685 kb).
Rights and permissions
About this article
Cite this article
Chen, S., Zhao, P., Jiang, L. et al. Cu2O-mediated assembly of electrodeposition of Au nanoparticles onto 2D metal-organic framework nanosheets for real-time monitoring of hydrogen peroxide released from living cells. Anal Bioanal Chem 413, 613–624 (2021). https://doi.org/10.1007/s00216-020-03032-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-03032-6