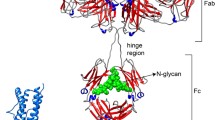
We employ a novel circular dichroism (CD) technology coupled with statistical analysis to assess the higher-order structure (HOS) similarity of commercially available trastuzumab and a proposed biosimilar. This technology shows good potential for enabling the comparison of similarities and differences in secondary and tertiary structures of proteins. Multiple CD spectra in far- and near-UV are obtained reproducibly from a CD spectrometer with fully integrated autosampler and flow cell to eliminate the errors typically associated with sample handling on manual CD spectrometers. The significance of similarities or differences was quantified statistically using three analytical methods (weighted spectral difference, correlation coefficient, and area of overlap). HOS comparison of the secondary structure (far-UV CD spectra) suggested similarity between the innovator drug, trastuzumab (Herceptin®), and biosimilar products. However, the tertiary structure (near-UV CD spectra) suggested statistically significant differences. These differences may be due to changes in tertiary structure or to modifications and degradations during the process of production or storage. The results show that the automated circular dichroism technology coupled with statistical analysis is a robust tool for enabling the comparison of similarities and differences in secondary and tertiary structures of protein products.
Similar content being viewed by others
References
H. Schellekens, Nature Biotechnol., 22, No. 11, 1357–1359 (2004).
S. D. Roger, Nephrology, 11, N 4, 341–346 (2006).
http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf (accessed 06.09.17.).
http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf (accessed 06.09.17.).
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf (accessed 06.09.17.).
https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM576786.pdf (accessed 05.22.18.).
S. Miao, L. Fan, L. Zhao, D. Ding, X. Liu, H. Wang, and W. S. Tan, Biomed. Res. Int., 7, 1–13 (2017).
J. Liu, T. Eris, C. Li, S. Cao, and S. Kuhns, BioDrugs, 30, No. 4, 321–338 (2016).
N. Seo, A. Polozova, M. Zhang, Z. Yates, S. Cao, H. Li, S. Kuhns, G. Maher, H. J. McBride, and J. Liu, mAbs, 10, No. 4, 678–691 (2018).
J. Visser, I. Feuerstein, T. Stangler, T. Schmiederer, C. Fritsch, and M. Schiestl, BioDrugs, 27, No. 5, 495–507 (2013).
J. Lee, H. A. Kang, J. S. Bae, K. D. Kim, K. H. Lee, K. J. Lim, M. J. Choo, and S. J. Chang, mAbs., 10, No. 4, 547–571 (2018).
N. J. Greenfield, Nat. Protoc., 1, No. 6, 2876–2890 (2006).
N. J. Greenfield, Nat. Protoc., 1, No. 6, 2891–2899 (2006).
N. J. Greenfield, Nat. Protoc., 1, No. 6, 2733–2741 (2006).
C. H. Li, X. Nguyen, L. Narhi, L Chemmalil, E. Towers S. Muzammil, J. Gabrielson, and Y. Jiang, J. Pharm. Sci., 100, 4642–4654 (2011).
B. M. Teska, C. Li, B. C. Winn, K. K. Arthur, Y. Jiang, and J. Gabrielson, Anal. Biochem., 434, No. 1, 153–165 (2013).
J. C. Lin, Z. K. Glover, and A. Sreedhara, J. Pharm. Sci., 104, No. 12, 4459–4466 (2015).
N. N. Dinh, B. C. Winn, K. K. Arthur, and J. P. Gabrielson, Anal. Biochem., 464, No. 4, 60–62 (2014).
S. J. Prestrelski, N. Tedeschi, T. Arakawa, and J. F. Carpenter, Biophys. J., 65, 661–671 (1993).
B. S. Kendrick, A. Dong, S. D. Allison, M. C. Manning, and J. F. Carpenter, J. Pharm. Sci., 85, 155–158 (1996).
Y. Du, A. Walsh, R. Ehrick, W. Xu, K. May, and H. Liu, mAbs, 4, No. 5, 578–585 (2012).
http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5E/Step4/Q5E_Guideline.pdf. (accessed 05.22.18.).
A. S. Rathore and R. Bhambure, Anal. Bioanal. Chem., 406, No. 26, 6569–6576 (2014).
F. Sörgel, A. Schwebig, J. Holzmann, S. Prasch, P. Singh, and M. Kinzig, BioDrugs, 29, No. 2, 123–131 (2015).
S. Brokx, L. Scrocchi, N. Shah, and J. Dowd, Biologicals, 48, 28–38 (2017).
O. Kwon, J. Joung, Y. Park, C. W. Kim, and S. H. Hong, Biologicals, 48, 101–108 (2017).
E. L. Shaltout, M. A. Al-Ghobashy, F. A. Fathalla, and M. Y. Salem, J. Pharm. Biomed. Anal., 97, 72–80 (2014).
O. Montacir, H. Montacir, M. Eravci, A. Springer, S. Hinderlich. A. Saadati, and M. K. Parr, J. Pharm. Biomed. Anal., 140, 239–251 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 87, No. 5, p. 850, September–October, 2020.
Rights and permissions
About this article
Cite this article
Fang, J., Li, H., Wu, S. et al. Higher-Order Structure Comparison of a Proposed Biosimilar and the Innovator Biotherapeutic Trastuzumab using Circular Dichroism Coupled with Statistical Analysis. J Appl Spectrosc 87, 938–945 (2020). https://doi.org/10.1007/s10812-020-01092-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-020-01092-1