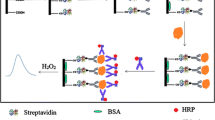
We consider the possibilities of applying the plasmon-enhanced fluorescence of monoclonal antibodies labelled with fluorescein isothiocyanate (FITC) to the prostate-specific antigen (PSA) for increasing the immunofluorescence analysis sensitivity. An immunochemical test system featuring two center binding using a pair of noncompeting monoclonal antibodies in combination with silver plasmon nanoparticles was used for the first time for determining the PSA concentration. The advantages over the standard PSA immunofluorescence assay are as follows. The intensity of the recorded fluorescent signal is enhanced by 2.3–3.2 times in the presence of silver nanoparticles in comparison with the signal of the test system on the intact surface of a polystyrene plate. The signal-to-noise ratio is increased by up to two times. In addition to the plasmon-enhanced fluorescence, the effect of an intermediate polyelectrolyte layer for enhancement of the adsorption capacity of the primary PSA antibodies is shown, which, in turn, affects the fluorescence signal intensity. Growth of the plasmon fluorescence enhancement factor with increasing concentration of the labelled antibodies indicates suppression of the self-quenching of the fluorescent labels by metal nanoparticles.
Similar content being viewed by others
References
V. S. Kamyshnikov, Oncomarkers: Methods of Determination, Reference Values, and Test Interpretation [in Russian], MED Press Inform, Moscow (2011).
V. S. Pervyi and V. F. Sukhoi, Oncomarkers: Clinical Diagnostic Handbook [in Russian], Feniks, RostovonDon, Russia (2012).
P. Karami, H. Khoshsafar, M. JohariAhar, F. Arduini, A. Afkhami, and H. Bagheri, Spectrochim. Acta A, 222, 117218 (2019).
P. Damborský, N. Madaboosi, V. Chu, J. P. Conde, and J. Katrlik, Chem. Papers, 69, 143–149 (2013).
F. Tan, Y. Yang, X. Xie, L. Wang, K. Deng, X. Xia, X. Yang, and H. Huang, Analyst, 143, 5038–5045 (2018).
H.-M. Kim, J.-H. Park, D. H. Jeong, H. Y. Lee, and S. K. Lee, Sens. Actuator BChem., 273, 891–898 (2018).
J. W. Attridge, P. B. Daniels, J. K. Deacon, G. A. Robinson, and G. P. Davidson, Biosens. Bioelectron., 6, 201–214 (1991).
F. Yu, B. Persson, S. Lofas, and W. Knoll, Anal. Chem., 76, 6765–6770 (2004).
K. Sokolov, G. Chumanov, and T. M. Cotton, Anal. Chem., 70, 3898–3905 (1998).
S. M. Tabakman, J. Lau, J. T. Robinson, J. Price, S. P. Sherlock, H. Wang, B. Zhang, Z. Chen, S. Tangsombatvisit, J. A. Jarrell, P. J. Utz, and H. Dai, Nat. Commun., 2, 466 (2011).
L. Zhou, F. Ding, H. Chen, W. Ding, W. Zhang, and S. Y. Chou, Anal. Chem., 84, 4489–4495 (2012).
R. Zhang, Z. Wang, C. Song, J. Yang, and Y. Cui, J. Fluoresc., 23, 551–559 (2013).
O. Kulakovich, N. Strekal, M. Artemyev, A. Stupak, S. Maskevich, and S. Gaponenko, Nanotechnol., 17, 5201–5206 (2006).
J. R. Lakowicz, J. Malicka, S. D'Auria, and I. Gryczynski, Anal. Biochem., 320, 13–20 (2003).
K. Okuda, Digest. Dis. Sci., 31, 133S–146S (1986).
Y. Wang, A. Brunsen, U. Jonas, J. Dostalek, and W. Knoll, Anal. Chem., 81, 9625–9632 (2009).
L.-H. Jin, S.-M. Li, and Y.-H. Cho, Biosens. Bioelectron., 33, 284–287 (2012).
H. Y. Song, T. I. Wong, A. Sadovoy, L. Wu, P. Bai, J. Deng, S. Guo, Y. Wang, W. Knoll, and X. Zhou, Lab. Chip., 15, 253–263 (2015).
T. Kaya, T. Kaneko, S. Kojima, Y. Nakamura, Y. Ide, K. Ishida, Y. Suda, and K. Yamashita, Anal. Chem., 87, 1797–1803 (2015).
Q. Zheng, L. Wu, T. I. Wong, J. Zhang, X. Liu, X. Zhou, P. Bai, B. Liedberg, and Y. Wang, Int. J. Nanomed., 12, 2307–2314 (2017).
O. S. Kulakovich, M. V. Artem’ev, A. P. Stupak, S. A. Maskevich, and S. V. Gaponenko, O. S. Kulakovich, M. V. Artem’ev, A. P. Stupak, S. A. Maskevich, and S. V. Gaponenko, J. Appl. Spectrosc., 73, 892–896 (2006).
D. V. Guzatov, S. V. Vaschenko, V. V. Stankevich, A. Ya. Lunevich, V. F. Glukhov, and S. V. Gaponenko, J. Phys. Chem. C, 116, 10723–10733 (2012).
A. A. Ramanenka, S. V. Vaschenko, V. V. Stankevich, A. Ya. Lunevich, Yu. F. Glukhov, and S. V. Gaponenko, J. Appl. Spectrosc., 81, 222–225 (2014).
S. Vaschenko, A. Ramanenka, O. Kulakovich, A. Muravitskaya, D. Guzatov, A. Lunevich, A. Ya. Lunevich, Y. F. Glukhov, and S. V. Gaponenko, Proc. Eng., 149, 57–66 (2016).
D. V. Guzatov, S. V. Gaponenko, and H. V. Demir, Plasmon., 13, 2133–2140 (2018).
P. C. Lee and D. Meisel, J. Phys. Chem., 86, 3391–3395 (1982).
S. V. Gaponenko, Introduction to Nanophotonics, CUP, Cambridge (2010), p. 465.
F. R. Aussenegg, A. Leitner, M. E. Lippitsch, H. Reinisch, and M. Riegler, Surf. Sci., 189/190, 935–945 (1987).
N. Strekal, A. Maskevich, S. Maskevich, J. C. Jardillier, and I. Nabiev, Biopolymers (Biospectroscopy), 57, 325–328 (2000).
K. Takahashi, M. Fukada, M. Kawai, and T. Yokochi, Immunolog. Method., 153, 67–71 (1992).
L. S. Epstein and J. K. Lunney, J. Immunolog. Method., 76, 63–72 (1985).
N. A. Stearns, S. Zhou, M. Petri, S. R. Binder, and D. S. Pisetsky, PLoS ONE, 11, e0161818 (2016).
F. Caruso, Adv. Mater., 13, 11–22 (2001).
A. Muravitskaya, O. Kulakovich, P. M. Adam, and S. Gaponenko, Phys. Status Solidi (b), 255, 1700491 (2018).
A. C. McGeachy, N. Dalchand, E. R. Caudill, T. Li, M. Doğangün, L. L. Olenick, H. Chang, J. A. Pedersen, and F. M. Geiger, Phys. Chem. Chem. Phys., 20, 10846–10856 (2018).
V. A. Galievsky, A. S. Stasheuski, and S. N. Krylov, Anal. Chem., 89, 11122–11128 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 87, No. 5, pp. 796–803, September–October, 2020.
Rights and permissions
About this article
Cite this article
Koktysh, I.V., Melnikova, Y.I., Kulakovich, O.S. et al. Highly Sensitive Immunofluorescence Assay of Prostate-Specific Antigen Using Silver Nanoparticles. J Appl Spectrosc 87, 870–876 (2020). https://doi.org/10.1007/s10812-020-01083-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-020-01083-2