Abstract
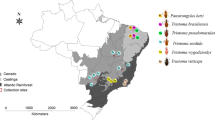
Direct test over the gut material from triatomine vectors and xenodiagnosis over mammalian hosts are classical techniques for Trypanosoma cruzi parasitological diagnosis. Nevertheless, negative results can be a source of uncertainty. Experimental models have allowed evaluating the tissue invasion of different strains of T. cruzi, but conventional techniques for tissue biopsies involve time-consuming and elaborated procedures and have low sensitivity. Gut material of collected triatomines (microscopically negative) (n = 114), material of mammal xenodiagnoses (microscopically negative) (n = 138), and biopsy material (microscopically negative) from experimentally infected animals (n = 34) with isolates from endemic areas of Chagas’ disease from Venezuela were used for DNA extraction and PCR for the amplification of kinetoplast DNA (kDNA) and satellite DNA (sDNA) of T. cruzi. Positive PCR was observed in 53.6% of collected triatomine material, 15.8% of parasitological negative xenodiagnosis material, and 70.6% in biopsies, revealing underestimation by the parasitological tests and the valour of this analysis with preserved material. Anzoátegui was the state with the highest percentage of infection, and the triatomine species Rhodnius prolixus and Panstrongylus geniculatus had the highest percentages of infection. Didelphis marsupialis and Canis familiaris were the most infected by T. cruzi revealed by PCR of xenodiagnosis material. In addition, the PCR technique allowed demonstrating the invasion of T. cruzi in all tissues analyzed, constituting a molecular marker of tissue invasion.

Similar content being viewed by others
References
Alkmim-Oliveira SM, Costa-Martins AG, Kappel HB, Correia D, Ramirez LE, Lages-Silva E (2013) Trypanosoma cruzi experimental congenital transmission associated with TcV and TcI subpatent maternal parasitemia. Parasitol Res 112:671–678
Añez N, Crisante G, Rojas A, Segninib S, Espinoza-Álvarez O, Teixeira MMG (2020) Update on Chagas’ disease in Venezuela during the period 2003–2018. Acta Trop 203:105310
Bazán PC, Lo Presti MS, Strauss M, Báez AL, Miler N, Paglini PA, Rivarola HW (2016) Quantitative PCR and unconventional serological methods to evaluate clomipramine treatment effectiveness in experimental Trypanosoma cruzi infection. Exp Mol Pathol 101:274–280
Bittencourt AL, Rodrigues de Freitas LA, Galvão de Araujo MO, Jácomo K (1981) Pneumonitis in congenital Chagas’ disease. A study of ten cases. Am J Trop Med Hyg 30:38–42
Cantillo-Barraza O, Garcés E, Gómez-Palacio A, Cortés L, Pereira A, Marcet P, Jansen A, Triana-Chávez O (2015) Eco-epidemiological study of an endemic Chagas disease region in northern Colombia reveals the importance of Triatoma maculata (Hemiptera: Reduviidae), dogs and Didelphis marsupialis in Trypanosoma cruzi maintenance. Parasit Vectors 8:1–10
Castro C, Santos MC, Silveira CA (2004) Comparative study between artificial xenodiagnosis performed immediately and four hours after venous punch. Rev Soc Bras Med Trop 37(2):128–130
Crisante G, Rojas A, Teixeira M, Añez N (2006) Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Trop 98:247–254
Ferrer E, Da Conceicão F, Campioli P, Lares M, López M, Rivera M, Viettri M, Medina M, Salcedo M, Morocoima A, Herrera L (2009) Validation of PCR protocols for the molecular diagnosis of Chagas’ disease. Salus 12:S163–S174
Ferrer E, Lares M, Viettri M, Medina M (2013) Comparison between immunological and molecular techniques for the diagnosis of Chagas disease. Enferm Infecc Microbiol Clin 31:277–282
Guarner J (2019) Chagas´ disease as example of a reemerging parasite. Semin Diagn Pathol 36:164–169
Lent H, Wygodzinsky P (1979) Revision of the Triatominae (Hemiptera, Reduviidae) and their significance as vectors of Chagas´ disease. Bull Am Mus Nat Hist 163:123–520
Morocoima A, Rodríguez M, Herrera L, Urdaneta-Morales S (2006) Trypanosoma cruzi: experimental parasitism of bone and cartilage. Parasitol Res 99:663–668
Morocoima A, Socorro G, Ávila R, Hernández A, Merchán S, Ortiz D, Primavera G, Chique J, Herrera L, Urdaneta-Morales S (2012) Trypanosoma cruzi: experimental parasitism in the central nervous system of albino mice. Parasitol Res 111:2099–2107
Moser D, Kirchhoff L, Donelson J (1989) Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol 27:1477–1482
Padilla CP, Alvarado U, Ventura G, Luna-Caipo D, Suárez M, Tuñoque JR, Ruelas-Llerena N, Fachín LA, Huiza A, Gonzáles L, Carranza JC, Vallejo GA, Cáceres AG (2017) Identifying Trypanosoma cruzi discreet typing units in triatomines collected in different natural regions of Perú. Biomédica 37:167–179
Rivera MG, Herrera L, Morocoima A, Aguilar C, Gárate T, López M, Lares M, Viettri M, Ferrer E (2015) Genetic variability of Trypanosoma cruzi TcI isolates from rural and urban areas of Venezuela. J Vector Borne Dis 52:23–29
Sá ARN, Kimoto KY, Steindel M, Grisard EC, Gomes ML (2018) Limit of detection of PCR/RFLP analysis of cytochrome oxidase II for the identification of genetic groups of Trypanosoma cruzi and Trypanosoma rangeli in biological material from vertebrate hosts. Parasitol Res 117:2403–2410
Saavedra M, Zulantay I, Apt W, Martínez G, Rojas A, Rodríguez J (2013) Chronic Chagas´ disease: PCR-xenodiagnosis without previous microscopic observation is a useful tool to detect viable Trypanosoma cruzi. Biol Res 46:295–298
Sales-Campos H, Kappel HB, Andrade CP, Lima TP, de Castilho A, Giraldo LE, Lages-Silva E (2015) Trypanosoma cruzi DTU TcII presents higher blood parasitism than DTU TcI in an experimental model of mixed infection. Acta Parasitol 60:435–441
Sambrook J, Russel D (2001) Molecular cloning: a laboratory manual, vol 6, III edn. Cold Spring Harbor, New York, pp 4–6.12
Savino W (2017) Endocrine immunology of Chagas’ disease. Front Horm Res 48:160–175
Silva-Dos-Santos D, Barreto-de-Albuquerque J, Guerra B, Moreira OC, Berbert LR, Ramos MT, Mascarenhas BAS, Britto C, Morrot A, Serra Villa-Verde DM, Garzoni LR, Savino W, Cotta-de-Almeida V, de Meis J (2017) Unraveling Chagas’ disease transmission through the oral route: gateways to Trypanosoma cruzi infection and target tissues. PLoS Negl Trop Dis 11(4):e0005507
Strauss M, Velázquez López DA, Moya DM, Bazán PC, Báez AL, Rivarola HW, Paglini-Oliva PA, Lo Presti MS (2018) Differential tissue distribution of Trypanosoma cruzi during acute experimental infection: further evidence using natural isolates. Mol Biochem Parasitol 222:29–33
Viettri M, Herrera L, Aguilar CM, Morocoima A, Reyes J, Lares M, Lozano-Arias D, García-Alzate R, Chacón T, Feliciangeli MD, Ferrer E (2018) Molecular diagnosis of Trypanosoma cruzi/Leishmania spp. coinfection in domestic, peridomestic and wild mammals of Venezuelan co-endemic areas. Vet Parasitol Reg Stud Rep 14:123–130
Viettri M, Herrera L, Aguilar CM, Morocoima A, Reyes J, Lares M, Lozano-Arias D, García-Alzate R, Chacón T, Feliciangeli MD, Ferrer E (2019) Molecular characterization of Trypanosoma cruzi/Leishmania spp. coinfection in mammals of Venezuelan co-endemic areas. J Vector Borne Dis 56(3):252–262
WHO (2020) Chagas’ disease (American trypanosomiasis) fact sheet. World Health Organization, Geneva http://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
Wincker P, Britto C, Borges-Pereira J, Cardoso M, Oelemann W, Morel C (1994) Use of simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am J Trop Med Hyg 51:771–777
Funding
This work has been partially supported by the following projects: Proyecto en Red Misión Ciencia MCT No. 2008000911-6 and No. 2007001442, Proyecto FONACIT No. G-2005000827, and PROYECTO LOCTI-Universidad de Carabobo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The project was approved by the Committee of Bioethics of the Institute of Biomedical Research of the University of Carabobo (BIOMED-UC), following the guidelines for human and animal care from the Commission of Bioethics of the Ministry of Science and Technology and the Operational Guidelines for Ethics Committees that Review Biomedical Research (TDR/PRD/ETHICS/2000.1). Informed consent was requested and signed by the participants and the owners of animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Herrera, L., Aguilar, C.M., Morocoima, A. et al. Detection of Trypanosoma cruzi DNA in false negative samples of collected triatomines, xenodiagnosis material, and biopsies of experimentally infected animals. Int Microbiol 24, 141–147 (2021). https://doi.org/10.1007/s10123-020-00149-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-020-00149-7