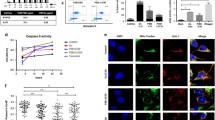
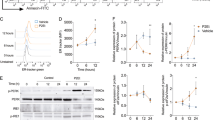
Abstract
P2Et extract obtained from the Caesalpinia spinosa plant is abundant in phenolic compounds such as gallic acid and ethyl gallate and can generate signals to activate the immune response by inducing a mechanism known as immunogenic cell death in murine models of breast cancer and melanoma. Immunogenic cell death involves mechanisms such as autophagy, which can be modulated by various natural compounds, including phenolic compounds with a structure similar to those found in P2Et extract. Here, we determine the role of autophagy in apoptosis and the generation of immunogenic signals using murine wild-type B16-F10 melanoma cells and cells with beclin-1 gene knockout. We show that P2Et extract and ethyl gallate induced autophagy, partially protecting tumor cells from death and promoting calreticulin exposure and the release of ATP. Although ethyl gallate showed a mechanism similar to that of P2Et, the induction of apoptosis and immunogenic signals was significantly weaker. In contrast, gallic acid-induced autophagy acted by blocking autophagic flux, which was associated with increased cell death. However, this compound did not induce any of the immunogenic death signals evaluated. Therefore, the complex extract has greater antitumor potential than isolated compounds. Here, we show that inducing autophagic flux with P2Et protects cancer cells from cell death and that this delay in cell death is required for the generation of immunogenic signals.






Similar content being viewed by others
References
Hasima N, Ozpolat B (2014) Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cáncer. Cell Death Dis 5(11):e1509–e1509
Liu H et al (2016) The natural occurring compounds targeting endoplasmic reticulum stress. Evid Based Complement Alternat Med. https://doi.org/10.1155/2016/7831282
Castañeda DM et al (2012) A gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line. BMC Complement Altern Med 12(1):38
Urueña C et al (2013) Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model. BMC Complement Altern Med 13(1):74
Gomez-Cadena A et al (2016) Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell Death Dis 7(6):e2243
Sandoval T et al (2016) Standarized extract from Caesalpinia spinosa is cytotoxic over cancer stem cells and enhance anticancer activity of doxorubicin. Am J Chin Med 44(8):1693–1717
Prieto K et al (2019) Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov 5:134
Focaccetti C et al (2019) Polyphenols as immunomodulatory compounds in the tumor microenvironment: Friends or Foes? Int J Mol Sci 20(7):1714
Galluzzi L et al (2016) Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17(2):97
Kepp O, Senovilla L, Kroemer G (2014) Immunogenic cell death inducers as anticancer agents. Oncotarget 5(14):5190–5191
Uruena C et al (2015) Multifunctional T Lymphocytes generated after therapy with an antitumor gallotanin-rich normalized fraction are related to primary tumor size reduction in a breast cancer model. Integr Cancer Ther 14(5):468–483
Gewirtz DA (2014) The four faces of autophagy: implications for cancer therapy. Cancer Res 74(3):647–651
Martins I et al (2013) Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ 21(1):79–91
Garg AD et al (2013) ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 9(9):1292–1307
Kimura S, Noda T, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3(5):452–460
Aits S et al (2015) Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11(8):1408–1424
Nicolau-Galmés F et al (2011) Terfenadine induces apoptosis and autophagy in melanoma cells through ROS-dependent and -independent mechanisms. Apoptosis 16(12):1253–1267
Mauthe M et al (2018) Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14(8):1435–1455
Michaud M et al (2011) Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334(6062):1573–1577
Junio HA et al (2011) Synergy-directed fractionation of botanical medicines: a case study with goldenseal (Hydrastis canadensis). J Nat Prod 74(7):1621–1629
Hubner A et al (2019) The synergistic behavior of antioxidant phenolic compounds obtained from winemaking waste’s valorization, increased the efficacy of a sunscreen system. Antioxidants (Basel) 8(11):530
Caesar LK, Cech NB (2019) Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep 36(6):869–888
Skroza D et al (2015) Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: a case of binary phenolic mixtures. J Food Compos Anal 38:13–18
Singh K et al (2014) Autophagic flux determines cell death and survival in response to Apo2L/TRAIL (dulanermin). Mol Cancer 13:70
Li DD et al (2016) Late-stage inhibition of autophagy enhances calreticulin surface exposure. Oncotarget 49:80842–80854
Salazar M et al (2011) Detecting autophagy in response to ER stress signals in cancer. Methods Enzymol 489:297–317
Wang Y et al (2020) Autophagy induction by thiostrepton improves the efficacy of immunogenic chemotherapy. J Immunother Cancer 8(1):e000462
Mileo AM, Miccadei S (2015) Polyphenols as modulator of oxidative stress in cancer disease: new therapeutic strategies. Oxid Med Cell Long. https://doi.org/10.1155/2016/6475624
Limonta P et al (2019) Role of endoplasmic reticulum stress in the anticancer activity of natural compounds. Int J Mol Sci 20(4):961
Acknowledgments
This project has the authorization of the Ministry of the environment, for the use of genetic resources through the Contract of Access to Genetic Resources No. 220/2018 for Colombian plant material. The authors want to express their gratitude to Paola Lasso for the edition of the figures.
Funding
Funding was provided by the Departamento Administrativo de Ciencia, Tecnología e Innovación COLCIENCIAS (120356934797, contract number 701–2013) and Vicerrectoría de Investigaciones, Pontificia Universidad Javeriana (007682 and 009067) Bogotá, Colombia. K.P was funded by the Departamento Administrativo de Ciencia, Tecnología e Innovación COLCIENCIAS, convocatoria 647 de 2014.
Author information
Authors and Affiliations
Contributions
KP carried out the experiments, analyzed data and co-wrote the paper. MPL carried out the lysosomal damage experiments. CU was involved in planning, analyzed data and supervised the work. CJA-D was involved in performed, planning and supervised the generation of knockout cell lines. AB and SF conceived the original idea supervised the project and prepared the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SF and CU are inventors of a granted patent related to P2Et. The remaining authors declare that the research was conducted in the absence of any potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prieto, K., Lozano, M.P., Urueña, C. et al. The delay in cell death caused by the induction of autophagy by P2Et extract is essential for the generation of immunogenic signals in melanoma cells. Apoptosis 25, 875–888 (2020). https://doi.org/10.1007/s10495-020-01643-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-020-01643-z