Abstract
The concept of nanomaterials membranes (NMs) promises to be a sustainable route to improve the membrane characteristics and enhance the performance of membrane bioreactors (MBRs) treating wastewater. This paper provided a critical review of recent studies on the use of membranes incorporating nanomaterials in membrane bioreactor (NMs-MBR) applications for wastewater treatment. Novel types of nanomaterials membranes were identified and discussed based on their structural morphologies. For each type, their design and fabrication, advances and potentialities were presented. The performance of NMs-MBR system has been summarized in terms of removal efficiencies of common pollutants and membrane fouling. The review also highlighted the sustainability and cost viability aspects of NMs-MBR technology that can enhance their widespread use in wastewater treatment applications.
Similar content being viewed by others
Introduction
Membrane bioreactors (MBRs) have proven to be one of the most effective technologies globally for treating wastewater from different sources1,2,3. In MBRs operation, wastewater treatment is carried out via a combination of biological unit (for the biodegradation of waste streams) and membrane filtration unit (for the separation of treated water from biosolids using membrane module). The two major categories of MBRs, based on their configuration and hydrodynamic control of membrane fouling, are submerged (SMBRs) and side stream MBRs. This technology was introduced >30 years ago and offers the advantages of a smaller footprint, high-quality treated water, less sludge production, low energy demand, and a higher removal rate for pollutants. Therefore, for obtaining high-quality treated wastewater, MBRs are recommended over other techniques, such as activated carbon adsorption, filtration, coagulation etc.; they can also be integrated with oxidation processes, such as photolysis, sonolysis and chemical/electrochemical oxidation for removal of micropollutants4,5,6,7. Thus, MBRs are well suited for on-site reuse of treated wastewater. As a consequence, driven by growing water scarcity, ageing infrastructure and increasingly stringent discharge norms, the MBR market is growing rapidly and has successfully contributed to the broader wastewater treatment market; furthermore, MBRs represent a significant market source for other membrane systems, especially microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and forward osmosis (FO)8,9,10.
Based on the literature, it is evident that in recent years, remarkable progress has been made in wastewater treatment via MBR technology. Wastewater reuse has been targeted11,12 and a range of industrial wastewaters have been tested13,14. Of particular interest are highly polluting effluents from sectors, such as tanneries15 and textiles16,17, as well as wastewaters with high salinity18. For example, textile wastewater containing azo dyes has been treated in a bioaugmented MBR coupled with a granular activated carbon (GAC) packed anaerobic zone resulting in over 95% dye removal in a short time19. Removal of micropollutants is also a focus area. For example, Trinh et al.20 investigated a full-scale MBR treating municipal wastewater for the removal of 48 trace organic chemical contaminants and found that removal percentage was over 90%. Katsou et al.21 illustrated removal of heavy metals from wastewater with removal efficiencies of Cu (II), Pb (II), Ni (II), and Zn (II) of 80%, 98%, 50%, and 77%, respectively. Mannina et al.22 reported an MBR pilot plant experimental study designed to treat saline wastewater contaminated with hydrocarbons, which resulted in a high total chemical oxygen demand (COD) removal of ~90%.
A plethora of studies have been done to enhance the performance of MBRs by integrating them with other systems10,23,24. In general, objectives of the integrated MBR systems are to improve permeate rates, decrease membrane fouling, increase process stability and to obtain treated wastewater of requisite quality. Laera et al.25 integrated an MBR with oxidation (either ozonation or a UV/H2O2 process) for the removal of organics and degradation of components in pharmaceutical wastewater. This integrated system was able to remove COD in the range of 85 − 95%, with complete removal of degraded products. De Jager et al.26 investigated color removal from textile wastewater using a pilot-scale, dual-stage MBR-Reverse osmosis (RO) system. The performance analysis revealed that total organic carbon (TOC) removal exceeded 80%, and in excess of 90% color removal efficiencies were recorded, potentially meeting the required discharge and reuse standards for wastewater. Phattaranawik et al.27 reported on a novel wastewater treatment process using membrane distillation bioreactor (MDBR) technology. This system has the potential to produce high-quality treated wastewater in one-step with capital and operation cost comparable to MBR coupled with RO.
In all MBR studies, membrane fouling is recognized as a major obstacle to the smooth operation and widespread application of this technology28,29,30,31. Fouling, in turn, requires membrane cleaning leading to higher operating costs due to additional energy, chemicals and system downtime. A variety of measures has been investigated for fouling control. To enhance performance, additives such as activated carbon have been used32. Deng et al.33 reported membrane fouling reduction using a sponge-submerged MBR (SSMBR); due to its higher zeta potential, particle size and relative hydrophobicity of the sludge flocs was increased. For economic feasibility, natural minerals have been used to obtain significant fouling reduction in terms of increased membrane permeability34. Variations in MBR operation such as moving bed MBR (MB-MBR) have been studied but are characterized by higher cake layer deposition and correspondingly higher irreversible fouling than in conventional MBR35,36. Izadi et al.37 reported reduced fouling with an integrated fixed bed MBR (FB-MBR). Membrane fouling control has been achieved with other strategies such as microbial fuel cell (MFC) MBR hybrids38, enzymatic quorum quenching for biofouling control39 and electro moving bed MBR (eMB-MBR) technology40. In particular, several studies have reported electrically-induced MBR technology for highly efficient wastewater treatment with significant membrane fouling reduction41,42,43,44,45.
To reduce fouling in MBR operation, the betterment of operational techniques in parallel with the design and development of improved membranes is imperative. Novel membranes for MBRs are especially promising in view of recent advances in nanomaterial-based membranes46,47,48,49,50 that offer leapfrogging opportunities to develop next-generation nanomaterials MBR (NMs-MBR) wastewater treatment technology. Many efforts have been made to embed various types of nanomaterials into membrane structures as doping materials to enhance membrane properties, such as hydrophilicity and antifouling characteristics and this approach has been used successfully in MBR technology51,52,53. A UK-based company located near Beacon Hill, Poole in Dorset, UK, is having contaminants removed via Werle’s MBR-coupled nanotechnology with improves landfill leachate quality and reduces COD, ammonia and solids significantly54. It is hoped that nanotechnology-enabled wastewater treatment promises to not only improve performance and offer affordable MBR wastewater treatment solutions but also to provide new treatment capabilities that could allow economic utilization of MBR facilities.
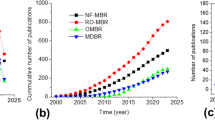
At the time of writing this manuscript, to the best of our knowledge, there are no reports documenting the influence of nanomaterials-based membranes in MBR technology for wastewater treatment. Hence, we present a critical review of the recent developments on the nanomaterials membrane bioreactor (NMs-MBR) technology for wastewater treatment in the current work and all existing papers have been covered. Based on structural characteristics and suitability for wastewater treatment, different types of nanomaterials membrane bioreactors can be classified as follows: (1) nanofibers membrane bioreactor (NFs-MBR)55,56, (2) nanoparticles membrane bioreactor (NPs-MBR)57,58, (3) nanotubes membrane bioreactor (NTs-MBR)59,60, (4) nanocrystals membrane bioreactor (NCs-MBR)61,62, (5) nanowires membrane bioreactor (NWs-MBR)63, and (6) nanosheets membrane bioreactor (NSs-MBR)64,65 (Fig. 1a). Growing recent publications related to the application of novel nanomaterials-based membrane in MBRs is evidence of increasing interest in this field (Fig. 1b) and historical development of nanomaterials membrane bioreactor technology has been depicted in Fig. 1c. The sustainability and cost-benefit analysis of NMs-MBR technology are also discussed in this work for broader application. Finally, the challenges and future perspectives of this new technology are provided.
a Examples of commonly used nanomaterials membrane bioreactor (NMs-MBR) technology (left to right) nanofibers membrane bioreactor (NFs-MBR), nanoparticles membrane bioreactor (NPs-MBR), nanotubes membrane bioreactor (NTs-MBR), nanocrystals membrane bioreactor (NCs-MBR), nanowires membrane bioreactor (NWs-MBR), nanosheets membrane bioreactor (NSs-MBR), and the advantages of using NMs-MBR technology are fouling control, high efficiency and sustainability. b Diagram of the total number of publications related to different types of nanomaterials membrane bioreactor (NMs-MBR) technology. Until 3rd August 2020, which were collected from the web of science scientific database, c Historical development of nanomaterials membrane bioreactor (NMs-MBR) technology for wastewater treatment. In 2005, Tae-Hyun Bae investigated the ability of TiO2-embedded nanocomposite membrane for membrane bioreactor (NPs-MBR), In 2009, Decostere Bjorge evaluated the electrospun nanofiber membrane for membrane bioreactor (NFs-MBR), In 2014, Chuanqi Zhao prepared nanosheets membrane and tested for membrane bioreactor (NSs-MBR), In 2015, Zahra Rahimi applied nanotubes membrane for membrane bioreactor (NTs-MBR), In 2018, Jinling Lv synthesized nanocrystal membrane and used for membrane bioreactor (NCs-MBR) and In 2019, Xiafei Yin established nanowires membrane and used for membrane bioreactor (NWs-MBR).
Fundamentals of NMs-MBR technology
Despite the advantages of MBRs with respect to a smaller footprint and better treated water quality, the technology is limited by membrane fouling, which results in flux deterioration and downtime for membrane cleaning. The conventional MBR market is dominated by polymeric membranes such as polyvinylidene difluoride (PVDF) and polyethersulfone (PES)66,67,68. Among the various strategies investigated to reduce membrane fouling, improvement in membrane properties is one method. Examples include the implementation of NMs-MBR technology and other NM-based membranes69. The design and development of NMs-MBR systems represent a breakthrough technology as NMs-MBR are expected to comprehensively address the fouling issue while maintaining high flux and treated effluent quality. The set-up would be similar to conventional MBRs coupling aerobic or anaerobic biological treatment and membrane filtration, as shown in a typical schematic representation of submerged (a) aerobic NMs-MBR (ANMs-MBR)70 and (b) anaerobic NMs-MBR (AnNMs-MBR)71 are shown in Fig. 2. NMs-based membranes are more effective than conventional membranes with respect to hydrophilicity, surface roughness, thermal stability, hydraulic stability, fouling control, higher water permeability, and higher selectivity due to their small surface pore size72,73. Because of the improved properties of NMs-based membranes, the system footprint can be further reduced and overall performance enhanced. The fabrication methods used for NM membranes focus mainly on the addition of NMs into the polymer support, but deposition of NMs on the surface of the membrane is also increasingly used50. To date, a variety of NM-based membranes, including nanofibres74, nanoparticles75, nanotubes76, nanocrystals77, nanowires78 and nanosheets79 have been used for water treatment applications.
A typical schematic representation of submerged (a) aerobic nanomaterials membrane bioreactor (ANMs-MBR). The feed tank was loaded with wastewater influent and the mixture was ensured for continuous flow. Then feed tank was connected into the bioreactor tank through a pump and a membrane module (where different types of nanomaterials membrane can be used) was placed in the bioreactor tank and aeration medium was created by an air compressor in order to obtained simultaneous aeration/filtration system. In addition, a pressure gauge was activated to close the suction pump flow and open the backwash channel when the range of transmembrane pressure out of standard and consequently fouling on the membrane surface appeared. Finally, the effluent tank collected clean water for further application. b Anaerobic nanomaterials membrane bioreactor (AnNMs-MBR). The feed tank loaded wastewater influent was pumped into the bioreactor with a membrane module (where different types of nanomaterials membrane can be used). In this case, the reactor was fully air locked, and the production of biogas amount was detected by a portable gas meter. Subsequently, clean water was storage at the effluent tank for further use.
NMs-MBR technology: performances and progress
The unprecedented success of NMs membrane is recognized as an alternative route to enhance the performance, including mitigation of fouling issues. Here, we summarize briefly the common types of NMs-MBR that have been used in wastewater treatment.
Nanofibers membrane bioreactor (NFs-MBR)
Generally, nanofibers (NFs) membranes contain fibers with diameters that are typically less than 100 nm80,81. NFs-based membranes offer a suitable platform for a variety of applications due to their extremely high aspect ratio, which helps them to interlock with each other in a subtle form. Of all the methods available for the fabrication of NFs membranes, electrospinning is the most common approach and is followed by several researchers and industries worldwide82,83. Electrospinning offers several advantages such as ease of operation, material selectivity, and low cost. Additionally, the fiber characteristics in terms of high porosity, surface-to-volume ratio and variable arrangements can be controlled84. The physical and chemical properties of electrospun NFs membranes can be easily adjusted for multi-purpose applications. In particular, several studies presented in the literature have shown that NFs membranes are highly effective adsorbents and catalysts85,86. For example, Sundaran et al.87 synthesized a novel polyurethane (PU)/graphene oxide (GO)-based electrospun NFs membrane for dye adsorption that could remove 95% methylene blue and 92% rhodamine B, respectively. Hosseini et al.88 prepared a novel montmorillonite (MMT) clay-chitosan/poly(vinyl alcohol) (PVA)-based electrospun NFs affinity membrane that removed 95% Basic Blue 41 (BB41) and was characterized by high flux and antifouling properties.
Motivated by the above mentioned potential of NFs membranes, researchers have coupled them with MBRs to create NFs-MBRs (Fig. 3). The use of electrospun NFs in MBRs was initiated by Bjorge et al.89 and different applications examined. First, the membrane was functionalized using silver nanoparticles for pathogen removal; the functionalized membrane showed significantly higher pathogen removal (63%) than the non-functionalized membrane (10%). Second, the flat-sheet electrospun NFs membrane was used for wastewater treatment in a lab-scale submerged MBR. The results showed high removal of turbidity (99%), total suspended solids (TSS) (99%), COD (94%) and ammonium (NH4+) (93%). Last, wastewater generated in a music festival was treated in the NFs-MBR and the removal of suspended solids (SS), COD, total nitrogen (TN), and total phosphorus (TP) were within discharge limits.
The synthesis of nanofibers membrane was typically carried out by using an electrospinning machine, whereby a selected amount of polymeric solution was injected on a syringe connected by a plastic tube and passed to a needle. Then, a high voltage generator was supplied and acquires a Taylor cone shape, leading to the formation of nanofibers membrane and collected on an alumina disk plate. Furthermore, the as-prepared membrane can be used in an MBR plant for improved permeate effluent quality.
Daels et al.90 reported a comparative study of electrospun NFs membranes in three MBR set-ups viz., an activated sludge MBR, an activated sludge MBR with a cationic polymer (MPE50) flux enhancer, and a trickling filter (TF)-MBR. In the presence of the trickling filter, the NFs-MBR possessed better performance in terms of reduced irreversible fouling due to simultaneous turbidity removal of 75% by the membrane and trickling filter. Bilad et al.55 prepared electrospun NFs membrane and compared them with conventional commercial membranes in a lab-scale MBR. Heat treatment of the NFs membranes prevented layered fouling on the membrane surface and the overall performance was comparable to commercial membranes. Kim et al.91 fabricated and explored the performance of flat-sheet electrospun NFs membrane using PVDF blended with polymethyl methacrylate (PMMA) for wastewater treatment in the laboratory and pilot-scale MBR systems. The prepared membrane surface was much smoother and had higher levels of porosity and permeability than a conventional cast membrane, indicating lower fouling. In pilot tests with secondary effluent from the wastewater treatment plant of the local zoo, COD removal (48%) was low but suspended solids removal was complete.
Nanoparticles (NPs) addition in NFs membranes has also been reported. Zhao et al.92 fabricated three-dimensional (3D) woven fabric filters decorated with silver nanoparticles doped polyacrylonitrile (PAN) NFs for wastewater treatment in an MBR. Microscopy of the used membrane showed that NFs-embedded fabric filter surface had few clusters of proteins, bacteria cells and polysaccharides. The silver nanoparticles doped NFs membranes showed 40–50% higher flux and substantially greater flux recovery than the undoped membranes. The results were attributed to the excellent antimicrobial property of the silver nanoparticles. Moradi et al.56 investigated the performance of PAN electrospun NFs membrane deposited with fumarate-alumoxane (FumA) nanoparticles for use in an MBR application. Incorporating low amounts of FumA nanoparticles improved surface hydrophilicity and reduced the irreversible fouling in terms of the highest flux recovery ratio of 96% and the lowest irreversible fouling rate of 4% for 2 wt% Fum-A addition.
NFs-based membranes have also been used in extractive MBRs (EMBR), which is based on combining an aqueous-aqueous extractive membrane process and biodegradation. For example, Wang’s group first applied polydimethylsiloxane (PDMS)-coated PVDF nanofibrous composite membrane for phenol removal in EMBR93. Results showed that after two weeks of operation, the mass transfer coefficient for phenol removal was stable at 4.1 ± 0.3 × 10−7 m.s−1; this was four times higher than with commercial silicone rubber. Subsequently, they developed a novel PDMS-coated NFs composite membrane with superhydrophobic or superhydrophilic surfaces with the better performance94. Among them, a nanofibrous membrane with superhydrophobic surface exhibited higher stability over 14 days for industrial wastewater treatment and demonstrated 10-times higher phenol extraction efficiency than that of the conventional PDMS membrane. On the other hand, superhydrophilic surface was covered by more polysaccharides and led to lower stability.
In another work, Shao’s group prepared PDMS/PMMA-based electrospun nanofiber membrane and used it in an EMBR system for phenol saline wastewater treatment95. High-mass transfer coefficient of phenol (8.8 × 10−7 m s−1) and high salt rejection (>99.96%) indicated high selectivity of salt/phenol. The same membrane was used in treating phenol-laden saline wastewaters in a novel external EMBR system96. High simultaneous removal of phenol (14.1–290.7 mg L−1) and ammonium (0.5–43.5 mg L−1) were achieved with a decrease in toxicity (6.3–70.5%).
The role of electrospun nanofiber membranes in anaerobic membrane bioreactors has been examined recently97. Results showed that during short-term filtration test, the as-prepared PVDF nanofiber membrane performed better than the commercial membrane in terms of low transmembrane pressure (TMP) with excellent flux retention. Additionally, suspended solids removal was over 99%, which was comparable to the commercial membrane. The membrane is currently being investigated on a full-scale anaerobic membrane bioreactor system. Table 1 summarizes NFs-MBR technology used in wastewater treatment.
Nanoparticles membrane bioreactor (NPs-MBR)
The use of NPs represents a promising direction for enhancing the performance of NMs-based media in wastewater treatment due to their large surface area and size- and shape-dependent properties. Additionally, they are suitable for functionalization with several chemical groups to augment their catalytic properties98,99,100. However, there are still some limitations to the widespread use of NPs in wastewater treatment applications. These include the separation of exhausted NPs from the treated water for reusability, their tendencies to aggregate in the system and the need for an in-depth understanding of the behavior and fate of NPs in the wastewater treatment systems101,102. Hence, it is necessary to develop supporting material that could help to maintain their performance. Membrane-based materials are playing a key role in the development of novel NPs-based membranes for effective wastewater treatment.
Recent attempts have shown that NPs-embedded membrane can be used to improve the performance of MBR systems (Fig. 4). Bae and Tak51 published a pioneering work on self-assembled titanium dioxide (TiO2) nanocomposite membrane that reduced fouling significantly in MBR wastewater treatment system. This work inspired many researchers to investigate NPs-MBR systems for wastewater treatment. For instance, Su et al.103 explored a similar approach using a TiO2 composite membrane in the MBR system. The presence of TiO2 NPs coated on the membranes improved the surface hydrophilicity and reduced membrane fouling than the virgin membrane. Liu et al.104 developed nano-TiO2/PVA polyester composite membrane with 10 μm pore size and tested in anoxic/oxic MBR systems. Incorporation of nano-TiO2 played a significant role in improving membrane performance in terms of lower fouling rate with higher pure water flux, as well as higher removal of contaminants compared to commercial PVDF membrane. Hu et al.105 applied nano-TiO2 on the PVDF membrane surface and tested in algal MBRs for wastewater treatment. The modified membrane showed the best removal efficiencies of P (78%) and N (34%); it had enhanced hydrophilicity and only 50% of the total resistance of the pristine membrane. Tavakolmoghadam et al.106 modified PVDF membrane surface by sputtered nano-TiO2 and applied in MBRs. The modified membrane had higher hydrophilicity and two-fold improvement in the filtration index compared to the pristine membrane. In addition, minimal leaching of nano-TiO2 particles occurred after washing, as confirmed by EDX analysis. Compared to pristine polypropylene membranes, polypropylene/TiO2 nanocomposite membranes used in MBR for treatment of oil refinery wastewater showed better antifouling characteristics107,108. The results demonstrated that presence of small amount of nano-TiO2 (0.75 wt%) enhanced the thermal and mechanical properties of the modified membrane besides increasing the critical flux (64 L m-2 h−1 for the modified membrane compared to 34.5 L m-2 h−1 for the pristine membrane).
The nanoparticles membrane was prepared according to the wet spinning technique. Typically, a certain amount of nanoparticles was gently added to the polymer solution bath and keep overnight for degas. Then, a mixed bath kept in the water bath another 24 h and dried of the as-prepared membrane to be loaded in the MBR tank for wastewater treatment.
Homayoonfal et al.109 examined polysulfone (PSf)/alumina nanocomposite membranes with the principal aim of reducing biofouling in the MBR system. Their filtration experiments demonstrated that the addition of alumina NPs increased the membrane hydrophilicity and resulted in 83% reduction in membrane fouling. In addition, 91% of dye rejection (DR) could be achieved, indicating that nanoparticles blended membranes enhanced MBR performance. A magnetic nanocomposite membrane influences MBR treatment performance, as reported by Mehrnia et al.53. They prepared a magnetic membrane by blending Fe3O4 NPs of size 60–70 nm into a PSf ultrafiltration membrane. The results demonstrate that nanocomposite membranes have 27% lower filtration resistance (Rf), 30% higher flux and led to 41% higher COD removal than a commercial membrane. Amini et al.110 introduced silica (SiO2) NPs-based high-density polyethylene (HDPE) membranes for the MBR system. They produced a flat-sheet type of membrane via a thermally induced phase separation (TIPS) approach. An increased amount of SiO2 NPs (0.5 wt.% and above) could enhance the membrane porosity. NPs addition controlled fouling with over 70% reduction in irreversible fouling ratio. Liang et al.111 tethered superhydrophilic silica NPs to poly(methacrylic acid) grafted PVDF membrane for fouling control in an MBR system. While the bare membrane showed rapid flux decline to ~40% of the initial flux, the functionalized membrane maintained ~56% of the initial flux and flux recovery upon cleaning was ~100%.
NPs incorporated membranes have also been investigated in the electric field attached MBRs. Li et al.112 synthesized and tested graphene (Gr)/PANi-phytic acid membrane in such a system. Even with a small amount of graphene (0.02 mg L−1), the membrane displayed good conductivity, antifouling and filtration properties. Liu et al.113 modified polyester filter cloth with Gr/polypyrrole (PPy) or GO/PPy and successfully investigated the antifouling property with yeast suspension. Application of electric field enhanced flux in the Gr/PPy modified membrane by 20% compared to 10% with the membrane modified by PPy alone.
Alsalhy et al.114 studied the effect of zinc oxide (ZnO) NPs on polyvinyl chloride (PVC) membrane performance for the treatment of hospital wastewater through MBR technology. The benefit of using ZnO NPs was that they acted as an antibiofouling material, thus overcoming the formation of a cake layer on the membrane surface. Addition of 0.1 g of ZnO NPs improved pure water permeability by 315% and a maximum flux recovery efficiency of 87% was obtained with the incorporation of 0.3 g of ZnO NPs. Functionalized NPs have also been incorporated in MBR membranes. Etemadi et al.115 prepared a dual functionalized nanodiamond (ND) using an amino group as well as polyethylene glycol (PEG) grafted onto a cellulose acetate (CA) nanocomposite membrane to improve its surface hydrophilicity and efficiency within the MBR system. Fouling recovery ratio of 95 % was obtained. Tizchang et al.116 prepared silanized nanodiamond (SND) NPs intercalated PSf membrane that was tested in an MBR system for wastewater treatment. The neat PSf membrane exhibited a higher contact angle (83.10o) and lower flux recovery ratio (FRR) (28.89%), while the functionalized membrane revealed higher FRR (58.93%) and improved hydrophilicity (contact angle 76.44o). In sequence, they reported improved FRR in their other study carried out by Kivi et al.117 wherein nanodiamond nanoparticles were modified using two approaches viz. thermal carboxylation (ND-COOH) and grafting with polyethylene glycol (ND-PEG) and then used in preparing HDPE composite membrane for enhanced MBR system performance. Membranes with 0.75 wt% ND-PEG were the best in terms of high flux and anti-fouling properties exhibiting FRR of 77.9% compared to 61.7% for the bare membrane.
Pirsaheb et al.118 fabricated a novel nanocomposite PES membrane using hydrophilic polycitrate-Alumoxane (PC-A) NPs and achieved better MBR performance. The addition of these hydrophilic PC-A NPs increased the surface hydrophilicity of the membrane resulting in a high antifouling ability; complete turbidity removal was also obtained. Mahmoudi et al.119 prepared silver-decorated GO (Ag-GO)/PES membrane for MBR application. For the modified membrane, hydrophilicity was significantly improved (39 ± 2.9˚) compared to the bare membrane (67 ± 3.59˚). Moreover, the pristine membrane operation stability was limited to 20 h during MBR run, while the modified membrane continued to operate for a longer period with effective resistance against fouling. Ahsani et al.57 fabricated PVDF nanocomposite membrane with immobilized Ag-SiO2 nanoparticles and tested it in a submerged MBR system treating real pharmaceutical wastewater. The nanocomposite membrane showed improved hydrophilicity and considerable antibiofouling ability with FRR of 76%, while the neat membrane surface exhibited more extracellular polymeric substance with a lower FRR of 58%. Behboudi et al.58 developed PVC/modified Ag NPs membrane; studies in an MBR system showed higher COD removal of 94% compared to 66% with the pristine membrane. Subsequently, they prepared and tested polyvinyl chloride/polycarbonate/modified silver NPs-based nanocomposite membrane120. The presence of nanoparticles enhanced the membrane performance in MBR system in terms of higher removal percentage of COD (98.1%) and increased flux recovery (97.2%). Table 2 summarizes NPs-MBR technology used for wastewater treatment.
Nanotubes membrane bioreactor (NTs-MBR)
Nanotubes (NTs), which are primarily carbon-based materials, have received significant attention for removal of contaminants from wastewater121,122. Their tunable properties, namely, high aspect ratios, large surface areas, easy functionalization and water transport, make them particularly attractive123,124. Compared to activated carbon, carbon NTs offer the advantages of excellent self-assembly on supporting materials via chemical vapor deposition and can be immobilized in membrane filters125. NTs-based membranes have been employed in MBR systems (NTs-MBR) to enhance the system performance (Fig. 5).
The successful preparation of nanotubes membrane ideally obtained through the use of polymer and carbon nanotubes mixed solution. In addition, carbon nanotubes could be functionalized and formed a casting solution with polymer, which was transferred to a glass plate by a sliding blade and generated a flat surface of the membrane. After that, the as-prepared membrane placed in the MBR module for treating wastewater.
Rahimi and co-workers52 designed a novel membrane using multi-walled carbon nanotubes (MWCNTs) blended PES for an MBR system. The carbon NTs were initially functionalized with amino groups before embedding in the PES substrate using the phase inversion method. Different concentrations of MWCNTs (0.05, 0.1 and 1 wt.%) were used and the modified MWCNTs exhibited excellent stability (up to 5 h). Among the different membranes, 0.1 wt.% loaded sample showed high porosity (89.3%) and low contact angle (52o), leading to the most permeable membrane with pure water flux of 106.75 kg/m2 h−1. This membrane showed higher bovine serum albumin (BSA) rejection (~60%) compared to the control (bare) membrane (~25%). The antifouling property was also improved with a FRR of 89.7% against 70% for the control; this was attributed to ionized amine groups on the modified MWCNTs surface bonding with a water layer thereby averting protein adsorption and consequent fouling.
In another study, Ayyaru et al.59 fabricated a novel PVDF membrane blended with CNTs, with and without sulfonation; these membranes were studied for sludge retention in a wastewater treatment plant via an MBR system. Though incorporation of CNTs improved the membrane properties compared to the pristine PVDF membrane, sulphonated CNTs were more effective compared to CNTs without functionalization. Incorporation of sulphonated CNTs increased membrane porosity (84%), enhanced hydrophilicity (contact angle of 51o) and improved protein (BSA) rejection (90%). The FRR (83.52%) was considerably higher than for the pristine PVDF membrane (50% FRR). Finally, the authors demonstrated that unlike the CNTs, the prepared modified membranes were non-toxic to bacteria.
Carbon nanotubes hollow fiber membranes (CNTs-HFMs) have also been developed and used in anaerobic electro-assisted membrane bioreactor60. The performance of this novel system was quantified at low temperature (15 − 20 °C) over an operation period of around 100-days. Good TMP recovery with a COD removal of over 95% was reported with the application of the electric field. Their next studies in anaerobic MBR with CNTs membrane in the presence of electric field126 showed a similar trend. It was noticed that after 120 min filtration, a relatively high membrane flux rate (412.2 L bar−1 m−2 h−1) was achieved for electric-assisted MBR while flux rate was halved (200.6 L bar−1 m–2 h−1) in the absence of electric field. The quality of treated effluent was good (COD < 50 mg L−1, NH4+-N < 2 mg L−1). Overall, the results strongly demonstrated that the presence of electric field could significantly alleviate the fouling plus enhance pollutants removal during MBR operation. Mulopo127 prepared CNTs-PSf nanocomposite membranes for use in an anaerobic MBR designed to treat the bleach effluent from pulp and paper industries. Permeability of the CNTs nanocomposite membrane was higher (0.6–0.7 L m−2 h−1) compared to the neat PSf membrane (0.15–0.25 L m−2 h−1); this is due to the O–H bonds that modifies membrane properties such as contact angle and roughness. However, COD and SS removal were similar with both membranes.
In another study by Khalid et al.128, CNTs were functionalized with PEG and used as nanofillers in the preparation of PSf nanocomposite membrane for wastewater treatment in MBR. They followed a non-solvent induced phase separation (NIPS) method. The prepared CNTs-PEG showed excellent dispersion stability even after 30 days without any agglomeration while the pure CNTs had poor dispersion stability of 1 day. The addition of varying amounts of CNTs-PEG (0.1–1.0 wt%) improved membrane hydrophilicity and water permeability. Best results were obtained with 0.25 wt% CNTs-PEG addition with increased hydrophilicity evinced by lower contact angle (57°) compared to 65° for pristine PSf membrane. The membrane porosity was higher—54% for CNTs-PEG PSf compared to 44% for pristine PSf. The permeability increased four-fold (from 4.41 L m–2 h−1 bar−1 for pristine PSf to 16.84 L m–2 h−1 bar−1 for CNTs-PEG PSf) and the FRR was enhanced as well (57% for pristine PSf against 80% for CNTs-PEG PSf). Table 3 presents the data of NTs-MBR technology used for wastewater treatment.
Nanocrystals membrane bioreactor (NCs-MBR)
Nanocrystals (NCs) are yet another form of NMs that have been considered for the development of nanocomposite membranes for wastewater treatment129. Among NCs, cellulose NCs (CNCs) are prime candidates as they are environmentally friendly, possess excellent thermal properties and are biodegradable130,131. Their superior mechanical properties, such as high tensile strength (~7 GPa) and high Young’s modulus (~130 GPa) allows them to be used widely as fillers132,133. Moreover, CNC surface is easily functionalized through different chemical moieties, which can improve the removal efficiency of specific pollutants. Addition of NCs to a membrane surface reportedly imbues the membrane with characteristics such as hydrophilicity, charge density and surface roughness, resulting in improved membrane performance134,135. Figure 6 shows the concept of NCs-based membranes in MBR systems (NCs-MBR).
The synthesis of nanocrystals was possibly composed by a mixed solution of polymer and nanopowder and acid hydrolysis step was followed. Then, it was added to the glass plate containing a polymeric support and phase inversion method acquires a nanocrystal membrane. Accordingly, the as-prepared membrane was fixed in the MBR membrane module in order to have a better quality of effluent.
In a recent study, a graphene oxide-cellulose nanocrystal (GO-CNC) composite/PVDF-based membrane was developed and tested for long-term MBR operation61. Compared to the pristine PVDF membrane (55% porosity and 0.3 µm pore size), the prepared GO-CNC/PVDF membrane showed higher porosity (80%) and larger mean pore size (1.2 µm); this translates to higher permeability and lower resistance to filtration. The pure water flux of GO-CNC/PVDF membrane was 3.2 times higher than the pristine PVDF membrane. The contact angle of the pristine PVDF membrane was 65.3°, whereas GO-CNC/PVDF membrane showed a much lower contact angle of 39.3° due to the strong H-bond interactions between the CNCs’ –OH groups and the GO sheets’ oxygen groups, thereby imparting better antifouling property to the membrane136. Surface charge (zeta potential) is another important membrane characteristic137. The zeta potential of GO-CNC/PVDF membrane (−17 mV) is twice that of the pristine PVDF membrane (−8 mV), suggesting that the foulants deposition on the membrane surface will be reduced because of the presence of more negatively charged GO-CNC composites. This was confirmed in MBR testing. For GO-CNC/PVDF membrane, one-time chemical cleaning was needed in 73 days in contrast to three chemical cleaning cycles required for the pristine PVDF membrane over this period. The cake layer thickness of 104.9 μm in the pristine PVDF membrane reduced to 41.38 μm for the GO-CNC/PVDF membrane indicating less foulants were deposited on the functionalized membrane surface significantly decreasing the fouling rate. Membrane fouling rate determined by measuring the relative flux after the cleaning process indicated that substantially irreversible fouling occurred on the unmodified membrane surface138 while the flux could be successfully recovered with the GO-CNC/PVDF membrane proving that the modified membrane had better antifouling property.
In another study, Li et al.62 developed Pd– reduced graphene oxide (rGO)/PVDF-carbon fiber cloth membrane and tested it for wastewater treatment in an MBR/MFC-coupled system. Here, Pd nanocrystal-rGO composite was synthesized and deposited on the surface of PVDF/carbon fiber through the electrodeposition process. The anti-fouling flux of the membranes was measured under two conditions viz. with and without application of electric field; the developed membrane functioned as a cathode. The presence of electric field enhanced the flux rate nearly two-fold 128–130 L m−2 h−1 compared to 66 L m−2 h−1 in the absence of electric field), indicating that membrane fouling could be controlled through the application of electric field during MBR operation. Removal of contaminants after 30 days of operation was stable with a high removal efficiency of COD (90%) and NH4+-N (81%) being achieved. The details are presented in Table 4.
Nanowires membrane bioreactor (NWs-MBR)
Within the family of NMs membranes, nanowire (NW) membranes have also emerged as materials with excellent mechanical, chemical, and thermal stabilities for use in wastewater treatment139,140. Nanowires offer higher aspect ratio (length to diameter/width ratio) of over 1000 compared to other one-dimensional nanomaterials such as nanofibers with an aspect ratio of 3–5; this makes nanowires useful in environmental applications141. The presence of NWs on a membrane surface enhanced pollutant removal and compared to conventional membranes, NW membranes exhibit flexible, uniform and multifunctional activity and could be used to remove other foulants such as microorganisms and trace organics142,143.
NWs-based membranes can be used in MBRs (NWs-MBR) to control membrane fouling and to achieve high treatment efficiency (Fig. 7). The only scientific article on NWs-MBR was published recently by Yin et al.63 where they prepared an innovative Cu-NW conductive microfiltration membrane and used it successfully in MBR application. Long-term operation was conducted at the presence of spontaneous electric field (SEF-MBR) to enhance the reduction in membrane fouling. In the initial 40 days, the membrane flux decreased and then became stable for both the Cu-NW membrane and commercial PVDF membrane. The SEF-MBR flux was 2.1 times higher than the control MBR with the commercial PVDF membrane; the fouling layer of around 80 μm on the Cu-NW membrane surface was thinner than on the PVDF membrane (179 μm). Monitoring the extracellular polymeric substances (EPS) on the membrane surface as an indicator of the extent of fouling showed that the total EPS amount was 62.0 mg/g VSS for the commercial PVDF membrane, while Cu-NW membrane surface had a lower total EPS content of 42.4 mg/g VSS. COD, total nitrogen and total phosphorous removal (94.5%, 78.5%, and 86.6%, respectively) were marginally better in the SEF-MBR when compared to the conventional MBR (92.7% COD, 70.5% total nitrogen and 80.4% total phosphorous removal). Overall, these results suggested that novel NMs-based membranes combined with an electric field could considerably enhance MBR performance. The details are presented in Table 4.
Nanowires membrane was also prepared by following the phase inversion method. A variety of polymeric materials and nanowires were gradually mixed to obtain a homogeneous solution and cast on non-woven support, which is termed as nanowires membrane. Finally, the as-prepared nanowires membrane was used in the MBR configurations for purifying water.
Nanosheets membrane bioreactor (NSs-MBR)
Nanosheets, two-dimensional (2D) NMs with atomic or molecular ratios, have received significant consideration as next-generation membrane materials for wastewater treatment144. Various kinds of nanosheet membranes, such as boron nitride (BN) nanosheet membranes145, GO nanosheet membranes146, titania nanosheet membranes147, molybdenum disulfide (MoS2) nanosheet membranes148 and graphitic carbon nitride (g-C3N4) nanosheet membranes149, have been used in water purification. Among these, the GO-based nanosheet membranes have demonstrated high efficiency due to their high charge density, different oxidation states and high mechanical strength.
Figure 8 displays the schematic for NSs-MBR system for wastewater treatment. In a recent study, Fathizadeh et al.64 prepared novel PES hollow fiber (HF) membranes coated with single-layer GO nanosheet (SLGO) and UV-treated SLGO and successfully tested these in MBR applications.The pure PES membrane surface roughness was 44 nm and after loading of SLGO, it decreased to 33 nm due to the UV-irradiation process. Also, the modified membrane exhibited excellent permeability (65 L m−2 h−1 bar−1) and the low fouling (<15% permeance reduction) compared to the pure PES membrane. A small amount of SLGO coating (6.2 mg m−2) was adequate to obtain 99% TOC removal in long-term MBR operation.The reason could be explained that the GO flakes were grafted by extra functional groups such as carboxyl or hydroxyl groups during UV-irradiation, which promoted the performances.
At first, GO nanosheet was prepared by adding a known amount of graphite powder and mixed with a polymeric solution. After sonication, a homogeneous solution was observed and placed on a non-woven cloth by a casting knife. Then, the formed membrane was placed in the water bath for 24 h for using in the MBR module. Moreover, clean water was collected for further use.
In 2014, Zhao et al.65 developed a novel composite microfiltration membrane by blending PVDF and GO nanosheets for MBR system. The GO-modified membrane showed higher hydrophilicity (contact angle of 60.50 ± 1.80o) than pristine PVDF membrane (contact angle of 78.30 ± 2.40o) because of the hydrophilic nature of GO, which actively increased the surface charge ratio of oxygen-containing groups on the membrane surface. Larger pore size (0.089 μm) and higher water permeability (552 L m−2 h−1 bar−1) was obtained compared to the pristine PVDF membrane (0.041 μm pore size and 171 L m−2 h−1 bar−1 water permeability). The critical flux (the flux above which deposition of particles or colloids occurs rapidly on the membrane surface forming cake or gel layer) was higher for the modified membrane (48–50 L m−2 h−1) compared to the pristine PVDF (30–33 L· L m−2 h−1). Concentration of EPS (17.87 g/m2) deposited on the pristine PVDF membrane surface was over three-time higher than with the modified membrane (5.97 g/m2). As a consequence of this lower fouling, cleaning frequency was reduced with the modified membrane exhibiting three-times longer filtration time than the PVDF membrane.
Ghalamchi et al.150 prepared a novel PES microfiltration membrane containing g-C3N4nanosheets/Ag3PO4 NPs through a phase inversion process for use in an MBR system. The addition of nanosheet improved the hydrophilicity of the prepared membrane compared to the bare PES membrane. Water flux was enhanced from 262 L m−2 h−1 to 360 L m−2 h−1 with a loading of 0.5 wt% nanosheet on the membrane surface. Moreover, the antifouling ability (determined with BSA filtration) was improved with an FRR of 57.5% for the nanosheet membrane; this was higher than the FRR of the bare PES membrane (39.1%).
Zinadini et al.151 synthesized GO nanosheet intercalated PES membranes with varying amounts of PES and GO for the treatment of milk process wastewater in an MBR system. Addition of GO decreased the contact angle and increased the water flux. A combination of 15 wt% PES loaded with 0.5 wt% GO was the most optimal in terms of high water flux and anti-fouling ability (FRR of 92.8%). The MBR performance improved at high mixed liquor suspended solids (MLSS) concentration of 14,000 mg/L with high flux (~30 kg m−2 h−1) and high removal of COD (95.4%), TN (66.8%) and TP (76.1%).
Beygmohammdi et al.152 studied the influence of different amounts (0–2 wt%) of GO and polyvinylpyrrolidone (PVP) grafted GO (PVP-GO) on the properties of pristine PVDF membrane in MBR application. Herein, PVP was successfully immobilized onto GO nanosheet through in-situ polymerization technique. The PVP-GO NPs so obtained had smaller particle size than the pristine GO nanosheets. Incorporation of 1.5 wt% GO or PVP-GO was optimal to obtain high hydrophilicity and pure water permeation. It is well known that the addition of GO improves membrane hydrophilicity153. Similar trend is observed in this study as well with the contact angle decreasing to 62.7° (PVP-GO/PVDF) and 74.8° (GO/PVDF)_from 105° for the neat PVDF membrane. The pure water flux, critical flux and FRR respectively are also higher (311.9 L m−2 h−1, 72.3 L m−2 h−1 and 80.81% for PVP-GO/PVDF; 205.2 L m−2 h−1, 41.5 L m−2 h−1, and 74.62% for GO/PVDF) compared to the neat PVDF membrane (85.2 L m−2 h−1, 19 L m−2 h−1, and 60.55%). In MBR operation, after 360 min filtration, 70% of the initial flux was maintained with PVP-GO/PVDF membrane compared to 49% with the neat PVDF membrane.
In another work, Wu et al.154 prepared PVP-GO/PVDF membrane through the chemical grafting approach and applied the membranes in algae-membrane photo-bioreactor (MPBR) systems for treating organic-rich wastewater. The PVP-GO/PVDF membrane demonstrated higher hydrophilicity (contact angle 62°) compared to the pristine PVDF membrane (contact angle 97°) and a remarkable improvement in pure water flux from 380 L m−2 h−1 (pristine membrane) to 614 L m−2 h−1 (PVP-GO/PVDF membrane). Moreover, contaminants removal efficiency in PVP-GO/PVDF–MBR (COD 97.8%, NH4-N+ 96.8%, NO3-N 76.9%) was higher than that in conventional PVDF-MBR (COD 93%, NH4-N+ 93.1%, NO3-N 70.6%). The details are presented in Table 4.
Membrane fouling
It is well recognized that membrane fouling in MBRs continues to be a major challenge136. Fouling in MBRs resulting from an interaction between the sludge components and the membrane material, can be either reversible or irreversible. While reversible fouling can be removed by applying physical treatments like air sparging and backflushing, chemical cleaning is needed to eradicate irreversible fouling. Reversible fouling always has a higher fouling rate than irreversible fouling155. From the foulant materials perspective, fouling can be classified under biological, organic and inorganic fouling. Biofouling caused by growth and deposition of biomass on the membrane surface is one of the most critical fouling problems in the long-term operation of MBRs. Organic fouling occurs due to deposition of organic matter, polysaccharides, lipids, proteins etc. while inorganic fouling refers to the accumulation of inorganic matter such as salts on the membrane surface156.
Specifically, several mechanisms are considered for membrane fouling phenomena in the MBRs operation29, such as solutes or colloids adsorption occurred within/on the membrane surface, cake layer formation and sludge flocs deposition on the membrane surface, foulants detachment due to shear forces and the temporal and spatial changes of foulants, i.e., the differences of biopolymer components and bacterial community in the cake layer appeared under the long-term MBRs operation. Typically, long-term MBRs operation carried out at constant flux with a variable TMP ranges. During the MBRs operation, an initial rise in TMP with a long-term weak increase TMP continued and third stage process formulated based on sudden TMP jump. It is known that sudden TMP increased observation called a severe membrane fouling condition in MBRs operation that causes higher critical flux than local flux; this necessitates membrane cleaning thereby disrupting MBR operation157,158. As the flux determines the membrane area required for the application, it has a strong influence on capital cost. Frequent membrane cleaning that might also reduce the membrane operational stability and lifespan thereby needing membrane replacement, adds up to the operational cost. Thus, approaches to control and minimize membrane fouling in MBRs continue to be researched extensively. Furthermore, contributing to membrane fouling are several factors such as membrane properties, operational conditions, feed components etc. so these have to be addressed comprehensively for fouling prevention in MBRs.
Accordingly, the preceding sections of this review paper have outlined how a variety of nanomaterials impregnated membranes that consistently exhibit enhanced hydrophilicity, can be utilized in controlling the fouling rate in NMs-MBRs operation. Figure 9 depicts how the integration of NMs membranes into the MBR process has been shown to be effective in improving antifouling behavior61. The strong interaction between the hydroxyl groups of CNC and hydroxyl, epoxide, carbonyl and carboxyl of GO facilitates the solution homogeneity and successful deposition on PVDF surface. At the initial stage of filtration, the commercial PVDF membrane can easily retain the suspended solids via the sieving mechanism due to the small pore size/porosity, resulting in irreversible fouling. With the more hydrophilic CNC/PVDF and GO-CNC/PVDF membranes, EPS, sludge flocs or microbes have less opportunity to attach with the membrane surface. Because of their hydrophilicity, which assists to adsorb water molecules on a large scale, the rate of adsorbents deposition is slower and results in a relatively thin cake layer on the membrane surface, Consequently, less membrane fouling occurs by using these membranes in long-term MBR operation61.
There are three types of membranes were chosen for understanding the fouling mechanism. Pristine PVDF membrane, the attachment of EPS, sludge flocs and suspended particles are higher than CNC/PVDF membrane, while a very few opportunities were observed for GO-CNC/PVDF membrane. More water molecules pass through the GO-CNC/PVDF membrane and less fouling occurred sequentially.
Sustainability and cost viability
The concept of sustainability in wastewater treatment has been of concern in the scientific community in recent years. In the literature, there are many studies available on the development of membranes incorporating NMs using green chemistry principles, but the application of such membranes in MBRs for wastewater treatment are not yet reported on a lab-scale/commercial scale. For example, Lv et al.159 fabricated electrospun nanofibrous PVA and konjacglucomannan (KGM)-based membranes loaded with ZnO nanoparticles with ecofriendly thermal cross-linking. These membranes were tested for environmental applications such as air filtration, photocatalytic degradation of dyes and bacteria. In another study, Ren et al.160 reported poly(lactic acid) (PLA) stereocomplex crystallite (SC)-based electrospun nanofibers loaded with an adsorbent obtained by the polymerization of tannic acid and hexamethylenediamine for \({\mathrm{Cr}}\left( {{\mathrm{VI}}} \right)\) removal. Environment-friendly plant-based components have also been examined. Copper oxide nanoparticles, produced by plant mediated green synthesis, have been incorporated in PES-CA nanocomposite membranes and plant extracts like curcumin in the nanomaterial form have been incorporated in PES membrane matrix136,137. The importance of nanomaterial choice has been emphasized with low-cost and non-toxic materials such as chitosan and iron-based nanomaterials being recommended based on sustainability aspects161.
From the literature, it is evident that while membranes incorporating NPs and to a lesser extent, NFs, have been investigated extensively for MBR applications, there are limited studies on membranes based on other NMs (NCs, NTs, NSs). This is possibly because NFs can be readily manufactured since electrospinning is a well-established process; also nanoparticles and CNTs are widely available commercially. Nanoparticles anchored on polymeric membranes and electrospun nanofiber membrane surface has demonstrated long-term permeability and better antifouling ability. Moreover, though NMs such as nanosponges, nanorods, and quantum dots-based membranes have been applied for wastewater treatment162,163, they have not yet been used in MBR applications. Among these, quantum dots-based membrane could be one of the most effective membranes for MBR applications due to better hydrophilicity, permeability and fouling resistance properties164.
The cost of NMs-based membranes needs to be established. Bjorge et al.89 estimated NFs membranes cost to be 20€/m2 that is cheaper than commercial membranes (50€/m2). Fatarella et al.165 estimated the production cost of NFs membranes to be 5€/m2, with the major cost component (75%) coming from the non-woven support. Indeed, the use of cheaper supports can reduce the total cost. This cost is much lower than the cost of traditional cast polymeric membranes, which run to 14–50€/m2 (refs. 9,90). However, the cost of raw materials for the development of NMs membranes can be quite varied. For example, reports suggest that clay-based NMs membranes enhance the pollutants removal capacity from wastewater in a sustainable way166,167 but there is a huge inequality in price between clays and some polymers (Suplementary Table S1). However, more detailed cost-benefit analyses are required for the future development of NMs-MBR systems.
Emerging applications of NMs-MBR technology
This review focuses on wastewater treatment applications of novel NMs-MBR technology. However, based on the previous success of conventional MBRs, NMs-MBR technology can also be employed in other emerging areas. The treatment of landfill leachates is one of the critical issues in environmental pollution control. A high concentration of organic and inorganic compounds, including micropollutants can be found in landfill leachates thereby threatening the quality of surface and groundwater sources. There have been a number of successful studies on the treatment of landfill leachates using MBR technology;168,169 thus, NMs-MBRs can be readily employed in this application54. Dabaghian et al.170 reported a comprehensive review of the potentiality of nanostructured membranes for landfill leachate treatment, and suggested that NMs membrane could lead to greater benefits than commercial membranes. Yet another prospect is in the area of anaerobic digestion (biomethanation) of organic wastes (including excreta) that includes the steps of hydrolysis, acidogenesis, acetogenesis and methanogenesis. Addition of nanomaterials helps to accelerate the digestion process, resulting in a high amount of volatile fatty acids (VFAs) and biogas being produced171,172,173. Instead of biogas production, alternatives such as hydrogen generation, recovery of VFAs for chemicals production etc. are being increasingly explored174,175. MBRs are being employed in this application176,177 and thus NMs-MBRs also have potential. With the increasing shift from waste treatment to resource recovery, nutrients (nitrogen and phosphorus) recovery is another key area; in this context, hybrid systems including MBR-based hybrids have been recommended178. With an emphasis on reducing energy use and recovering resources, anaerobic MBRs are being investigated extensively179. NMs-MBRs especially have a role in this sector considering the more serious membrane fouling that is encountered in anaerobic MBR systems158.
Challenges and future perspective
In spite of the progress in the application of nanomaterials-based MBR systems for wastewater treatment, there are challenges to be addressed for accelerating the practical application of NMs-MBR technology. It is necessary to justify the possible environmental threats that may be associated with this technology. Leaching of nanomaterials into aquatic environments can occur during long-term operation of such NMs-MBR, during the membrane synthesis process itself as well as due to inappropriate disposal of used membranes. The nanomaterials so released can undergo an environmental transformation, be taken-up by various aquatic organisms and could potentially pose a risk to both human health and environmental systems180,181. Assessment of nanomaterials membrane stability and understanding the dynamics of the release of nanomaterials from the membrane matrix is, therefore, a crucial part of the long-term NMs-MBR operation; besides the environmental implications, leaching of nanomaterials from the membrane surface could adversely impact membrane performance and its lifespan. For instance, the antimicrobial properties of the membrane could be improved by the addition of silver nanoparticles as biocidal agents but if continuously leached into water bodies, the desirable properties would not be achieved48,182,183.
Yet another challenge is the large-scale manufacture of the NMs membranes. Various techniques such as roll-to-roll, phase inversion, interfacial polymerization, stretching, track-etching and electrospinning process are in use. The roll-to-roll technique is still preferable for manufacturing of membranes, but it is a time-consuming process with low selectivity and difficulty in membrane pore size tuning184. Phase inversion and interfacial polymerization are widely used membrane fabrication techniques to obtain low-surface roughness, which is of primary interest to develop low-fouling membranes. However, phase inversion and interfacial polymerization induced membranes have low water permeability that would have to be enhanced through functionalization approaches185. The stretching method is useful to fabricate hydrophobic membranes but organic and biofouling propensity restricts its application. The track-etched method-based membrane offers some unique features such as low-surface roughness, but the low flux due to small porosity and high cost makes them less attractive186. Electrospinning technique is increasingly becoming popular as it offers a simple preparation method and different sizes of membranes can be produced; the challenge lies in jet instability, use of toxic solvents and adjustment of various operation parameters that are required to obtain membranes of adequate quality89,187.
According to our knowledge, many companies have started large-scale production of membranes/filters incorporating nanomaterials targeted at various applications (Table 5). Though this is not exactly what we proposed (use of such membranes in MBRs), considering the widespread applications of nanomaterials-based membranes/filters in water and wastewater segment, it can be expected that the MBR segment will subsequently be covered comprehensively by such products.
In short, it is imperative to find out the best possible solutions for the NMs-MBRs technology by mitigating the challenges as described previously. Efforts to prevent leaching of nanomaterials would involve more robust methods of fixing the nanomaterials on the membrane matrix such as chemical grafting. Advanced fabrication options that are both scalable and cost-effective need to be investigated; in this context additive (3D) manufacturing holds promise188. Additionally, operation parameters, feed characteristics and reactor configurations need to be suitably adjusted with effective protocols for NMs-MBR systems. In particular, maintenance practices including cleaning procedures have to be evolved and implemented regularly to control the operating cost and output. Consequently, the assessment of techno-economic analysis is one of the essential parameters that must be attempted in NMs-MBRs technology. This process could be interpreted by three indicators viz. economic viability, technical feasibility and environmental sustainability since it is very important for bioenergy and biobased products189. There is no techno-economic analysis data available specifically on NMs-MBRs technology, but it can follow models for conventional MBRs, which have been published in recent years190,191,192. It is of importance to control fouling by adopting different strategies. Lab-scale to real-scale transition must be started by maintaining the potential benefits such as sustainability and energy consumption. These aspects would, therefore, be key in promoting NMs-MBR technology for wastewater treatment.
Conclusion
This paper has critically reviewed the progress in the development of NMs-MBR applications for wastewater treatment. The possibility of synthesizing nanomaterials incorporated novel membranes with specific properties opens up opportunities for their use in MBR systems for wastewater treatment. While the use of NMs-MBR technology demonstrates good performance in terms of lower fouling and enhanced removal efficiency of pollutants, there are several aspects that are poorly understood presently. These include the cost of large-scale manufacture of such membranes, their lifespan in full-scale applications, the possibility of leaching of the nanomaterials in the wastewater/sludge etc. These issues should be further investigated before integration of nanomaterials membranes with MBRs, which will assist in designing next-generation MBRs technologies.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request (Prof. Vincenzo Naddeo, V.N.).
Code availability
No code was attempted or used during the current manuscript.
References
Judd, S. J. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem. Eng. J. 305, 37–45 (2016).
Shin, C. & Bae, J. Current status of the pilot-scale anaerobic membrane bioreactor treatments of domestic wastewaters: a critical review. Bioresour. Technol. 247, 1038–1046 (2018).
Hamedi, H., Ehteshami, M., Mirbagheri, S. A., Rasouli, S. A. & Zendehboudi, S. Current status and future prospects of membrane bioreactors (MBRs) and fouling phenomena: a systematic review. Can. J. Chem. Eng. 97, 32–58 (2019).
Li, Y.-Z., He, Y.-L., Liu, Y.-H., Yang, S.-C. & Zhang, G.-J. Comparison of the filtration characteristics between biological powdered activated carbon sludge and activated sludge in submerged membrane bioreactors. Desalination 174, 305–314 (2005).
Gurung, K., Ncibi, M. C., Shestakova, M. & Sillanpää, M. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode. Appl. Catal. B-Environ. 221, 329–338 (2018).
Nguyen, L. N., Hai, F. I., Kang, J., Price, W. E. & Nghiem, L. D. Removal of emerging trace organic contaminants by MBR-based hybrid treatment processes. Int. Biodeter. Biodegr. 85, 474–482 (2013).
Sathya, U., Nithya, M. & Balasubramanian, N. Evaluation of advanced oxidation processes (AOPs) integrated membrane bioreactor (MBR) for the real textile wastewater treatment. J. Environ. Manag. 246, 768–775 (2019).
Arévalo, J. et al. Wastewater reuse after treatment by tertiary ultrafiltration and a membrane bioreactor (MBR): a comparative study. Desalination 243, 32–41 (2009).
Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment (Elsevier, Oxford, 2011).
Neoh, C. H., Noor, Z. Z., Mutamim, N. S. A. & Lim, C. K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 283, 582–594 (2016).
Hai, F. I., Yamamoto, K. & Lee, C.-H. Membrane Biological Reactors: Theory, Modeling, Design, Management and Applications to Wastewater Reuse. Ch. 1 (IWA Publishing, London, 2019).
Falizi, N. J. et al. Evaluation of MBR treated industrial wastewater quality before and after desalination by NF and RO processes for agricultural reuse. J. Water Process. Eng. 22, 103–108 (2018).
Lin, H. et al. Membrane bioreactors for industrial wastewater treatment: a critical review. Crit. Rev. Env. Sci. Tec. 42, 677–740 (2012).
Mutamim, N. S. A., Noor, Z. Z., Hassan, M. A. A. & Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: a performance review. Desalination 305, 1–11 (2012).
Wang, Z. Encyclopedia of Membranes (Springer, Berlin, 2015).
Jegatheesan, V. et al. Treatment of textile wastewater with membrane bioreactor: a critical review. Bioresour. Technol. 204, 202–212 (2016).
Sun, F., Sun, B., Hu, J., He, Y. & Wu, W. Organics and nitrogen removal from textile auxiliaries wastewater with A2O-MBR in a pilot-scale. J. Hazard. Mater. 286, 416–424 (2015).
Tan, X., Acquah, I., Liu, H., Li, W. & Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 220, 1150–1162 (2019).
Hai, F. I., Yamamoto, K., Nakajima, F. & Fukushi, K. Bioaugmented membrane bioreactor (MBR) with a GAC-packed zone for high rate textile wastewater treatment. Water Res. 45, 2199–2206 (2011).
Trinh, T. et al. Removal of trace organic chemical contaminants by a membrane bioreactor. Water Sci. Technol. 66, 1856–1863 (2012).
Katsou, E., Malamis, S. & Loizidou, M. Performance of a membrane bioreactor used for the treatment of wastewater contaminated with heavy metals. Bioresour. Technol. 102, 4325–4332 (2011).
Mannina, G., Cosenza, A., Di Trapani, D., Capodici, M. & Viviani, G. Membrane bioreactors for treatment of saline wastewater contaminated by hydrocarbons (diesel fuel): an experimental pilot plant case study. Chem. Eng. J. 291, 269–278 (2016).
Goswami, L. et al. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: a review. J. Water Process. Eng. 26, 314–328 (2018).
Sert, G. et al. Investigation of mini pilot scale mbr-nf and mbr-ro integrated systems performance—preliminary field tests. J. Water Process. Eng. 12, 72–77 (2016).
Laera, G. et al. Removal of organics and degradation products from industrial wastewater by a membrane bioreactor integrated with ozone or UV/H2O2 treatment. Environ. Sci. Technol. 46, 1010–1018 (2011).
De Jager, D., Sheldon, M. & Edwards, W. Colour removal from textile wastewater using a pilot-scale dual-stage MBR and subsequent RO system. Sep. Purif. Technol. 135, 135–144 (2014).
Phattaranawik, J., Fane, A. G., Pasquier, A. C. & Bing, W. A novel membrane bioreactor based on membrane distillation. Desalination 223, 386–395 (2008).
Le-Clech, P., Chen, V. & Fane, T. A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 284, 17–53 (2006).
Meng, F. et al. Recent advances in membrane bioreactors (MBRs): membrane fouling and membrane material. Water Res. 43, 1489–1512 (2009).
Iorhemen, O. T., Hamza, R. A. & Tay, J. H. Membrane fouling control in membrane bioreactors (MBRs) using granular materials. Bioresour. Technol. 240, 9–24 (2017).
Iorhemen, O., Hamza, R. & Tay, J. Membrane bioreactor (MBR) technology for wastewater treatment and reclamation: membrane fouling. Membranes 6, 33 (2016).
Alighardashi, A., Pakan, M., Jamshidi, S. & Shariati, F. P. Performance evaluation of membrane bioreactor (MBR) coupled with activated carbon on tannery wastewater treatment. Membr. Water Treat. 8, 517–528 (2017).
Deng, L. et al. A comparison study on membrane fouling in a sponge-submerged membrane bioreactor and a conventional membrane bioreactor. Bioresour. Technol. 165, 69–74 (2014).
Malamis, S., Andreadakis, A., Mamais, D. & Noutsopoulos, C. Comparison of alternative additives used for the mitigation of membrane fouling in membrane bioreactors. Desalin. Water Treat. 52, 5740–5747 (2014).
Di Trapani, D., Di Bella, G., Mannina, G., Torregrossa, M. & Viviani, G. Comparison between moving bed-membrane bioreactor (MB-MBR) and membrane bioreactor (MBR) systems: influence of wastewater salinity variation. Bioresour. Technol. 162, 60–69 (2014).
Yang, S., Yang, F., Fu, Z. & Lei, R. Comparison between a moving bed membrane bioreactor and a conventional membrane bioreactor on organic carbon and nitrogen removal. Bioresour. Technol. 100, 2369–2374 (2009).
Izadi, A., Hosseini, M., Darzi, G. N., Bidhendi, G. N. & Shariati, F. P. Performance of an integrated fixed bed membrane bioreactor (FBMBR) applied to pollutant removal from paper-recycling wastewater. Water Resour. Ind. 21, 100111 (2019).
Wang, J. et al. Evaluation of energy-distribution of a hybrid microbial fuel cell–membrane bioreactor (MFC–MBR) for cost-effective wastewater treatment. Bioresour. Technol. 200, 420–425 (2016).
Oh, H.-S. et al. Control of membrane biofouling in MBR for wastewater treatment by quorum quenching bacteria encapsulated in microporous membrane. Environ. Sci. Technol. 46, 4877–4884 (2012).
Millanar-Marfa, J. et al. Fouling mitigation and wastewater treatment enhancement through the application of an electro moving bed membrane bioreactor (eMB-MBR). Membranes 8, 116 (2018).
Giwa, A. & Hasan, S. Theoretical investigation of the influence of operating conditions on the treatment performance of an electrically-induced membrane bioreactor. J. Water Process. Eng. 6, 72–82 (2015).
Giwa, A. & Hasan, S. W. Numerical modeling of an electrically enhanced membrane bioreactor (MBER) treating medium-strength wastewater. J. Environ. Manag. 164, 1–9 (2015).
Ahmed, M., Elektorowicz, M. & Hasan, S. W. GO, SiO2, and SnO2 nanomaterials as highly efficient adsorbents for Zn2+ from industrial wastewater—a second stage treatment to electrically enhanced membrane bioreactor. J. Water Process. Eng. 31, 100815 (2019).
Garcia, D. T. et al. Photocatalytic ozonation under visible light for the remediation of water effluents and its integration with an electro-membrane bioreactor. Chemosphere 209, 534–541 (2018).
Ahmed, M. A. & Hasan, S. W. Fe and Zn removal from steel making industrial wastewater by electrically enhanced membrane bioreactor. Desalin. Water Treat. 93, 9–21 (2017).
Daer, S., Kharraz, J., Giwa, A. & Hasan, S. W. Recent applications of nanomaterials in water desalination: a critical review and future opportunities. Desalination 367, 37–48 (2015).
Ibrahim, Y., Abdulkarem, E., Naddeo, V., Banat, F. & Hasan, S. W. Synthesis of super hydrophilic cellulose-alpha zirconium phosphate ion exchange membrane via surface coating for the removal of heavy metals from wastewater. Sci. Total. Environ. 690, 167–180 (2019).
Akther, N., Phuntsho, S., Chen, Y., Ghaffour, N. & Shon, H. K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 584, 20–45 (2019).
Giwa, A. et al. Selectivity of nanoporous MnO2 and TiO2 membranes for residual contaminants in treated wastewater. Chem. Eng. Technol. 41, 413–420 (2018).
Pendergast, M. M. & Hoek, E. M. A review of water treatment membrane nanotechnologies. Energ. Environ. Sci. 4, 1946–1971 (2011).
Bae, T.-H. & Tak, T.-M. Preparation of TiO2 self-assembled polymeric nanocomposite membranes and examination of their fouling mitigation effects in a membrane bioreactor system. J. Membr. Sci. 266, 1–5 (2005).
Rahimi, Z., Zinatizadeh, A. & Zinadini, S. Preparation of high antibiofouling amino functionalized MWCNTs/PES nanocomposite ultrafiltration membrane for application in membrane bioreactor. J. Ind. Eng. Chem. 29, 366–374 (2015).
Mehrnia, M. R. & Homayoonfal, M. Fouling mitigation behavior of magnetic responsive nanocomposite membranes in a magnetic membrane bioreactor. J. Membr. Sci. 520, 881–894 (2016).
Robinson, T. Membrane bioreactors: nanotechnology improves landfill leachate quality. Filtr. Separat. 44, 38–39 (2007).
Bilad, M. R., Westbroek, P. & Vankelecom, I. F. Assessment and optimization of electrospun nanofiber-membranes in a membrane bioreactor (MBR). J. Membr. Sci. 380, 181–191 (2011).
Moradi, G., Zinadini, S., Rajabi, L. & Dadari, S. Fabrication of high flux and antifouling mixed matrix fumarate-alumoxane/PAN membranes via electrospinning for application in membrane bioreactors. Appl. Surf. Sci. 427, 830–842 (2018).
Ahsani, M., Hazrati, H., Javadi, M., Ulbricht, M. & Yegani, R. Preparation of antibiofouling nanocomposite PVDF/Ag-SiO2 membrane and long-term performance evaluation in the MBR system fed by real pharmaceutical wastewater. Sep. Purif. Technol. 249, 116938 (2020).
Behboudi, A., Jafarzadeh, Y. & Yegani, R. Enhancement of antifouling and antibacterial properties of PVC hollow fiber ultrafiltration membranes using pristine and modified silver nanoparticles. J. Environ. Chem. Eng. 6, 1764–1773 (2018).
Ayyaru, S., Pandiyan, R. & Ahn, Y.-H. Fabrication and characterization of anti-fouling and non-toxic polyvinylidene fluoride-sulphonated carbon nanotube ultrafiltration membranes for membrane bioreactors applications. Chem. Eng. Res. Des. 142, 176–188 (2019).
Yang, Y., Qiao, S., Jin, R., Zhou, J. & Quan, X. Novel anaerobic electrochemical membrane bioreactor with a CNTs hollow fiber membrane cathode to mitigate membrane fouling and enhance energy recovery. Environ. Sci. Technol. 53, 1014–1021 (2019).
Lv, J., Zhang, G., Zhang, H. & Yang, F. Graphene oxide-cellulose nanocrystal (GO-CNC) composite functionalized PVDF membrane with improved antifouling performance in MBR: Behavior and mechanism. Chem. Eng. J. 352, 765–773 (2018).
Li, Y. et al. The performance of Pd-rGO electro-deposited PVDF/carbon fiber cloth composite membrane in MBR/MFC coupled system. Chem. Eng. J. 365, 317–324 (2019).
Yin, X., Li, X., Wang, X., Ren, Y. & Hua, Z. A spontaneous electric field membrane bioreactor with the innovative Cu-nanowires conductive microfiltration membrane for membrane fouling mitigation and pollutant removal. Water Environ. Res. 91, 780–787 (2019).
Fathizadeh, M. et al. Antifouling UV-treated GO/PES hollow fiber membrane in membrane bioreactor (MBR). Environ. Sci.: Water Res. Technol. 5, 1244–1252 (2019).
Zhao, C., Xu, X., Chen, J., Wang, G. & Yang, F. Highly effective antifouling performance of PVDF/graphene oxide composite membrane in membrane bioreactor (MBR) system. Desalination 340, 59–66 (2014).
Santos, A. & Judd, S. The commercial status of membrane bioreactors for municipal wastewater. Sep. Sci. Technol. 45, 850–857 (2010).
Krzeminski, P., Leverette, L., Malamis, S. & Katsou, E. Membrane bioreactors–a review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 527, 207–227 (2017).
Li, P. et al. Identify driving forces of MBR applications in China. Sci. Total. Environ. 647, 627–638 (2019).
Saikia, J., Gogoi, A. & Baruah, S. Environmental Nanotechnology. Ch. 7 (Springer, Cham, 2019).
Mirbagheri, S. A., Bagheri, M., Bagheri, Z. & Kamarkhani, A. M. Evaluation and prediction of membrane fouling in a submerged membrane bioreactor with simultaneous upward and downward aeration using artificial neural network-genetic algorithm. Process. Saf. Environ. 96, 111–124 (2015).
Amouamouha, M. & Badalians Gholikandi, G. Assessment of anaerobic nanocomposite membrane bioreactor efficiency intensified by biogas backwash. Chem. Eng. Process. Process. Intensif. 131, 51–58 (2018).
Kim, J. & Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 158, 2335–2349 (2010).
Qu, X., Brame, J., Li, Q. & Alvarez, P. J. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc. Chem. Res. 46, 834–843 (2012).
Liao, Y., Loh, C.-H., Tian, M., Wang, R. & Fane, A. G. Progress in electrospun polymeric nanofibrous membranes for water treatment: fabrication, modification and applications. Prog. Polym. Sci. 77, 69–94 (2018).
Lakhotia, S. R., Mukhopadhyay, M. & Kumari, P. Cerium oxide nanoparticles embedded thin-film nanocomposite nanofiltration membrane for water treatment. Sci. Rep. 8, 4976 (2018).
Dong, Y. et al. Stable superhydrophobic ceramic-based carbon nanotube composite desalination membranes. Nano. Lett. 18, 5514–5521 (2018).
Rafieian, F., Jonoobi, M. & Yu, Q. A novel nanocomposite membrane containing modified cellulose nanocrystals for copper ion removal and dye adsorption from water. Cellulose 26, 3359–3373 (2019).
Tan, X. et al. Novel AgNWs-PAN/TPU membrane for point-of-use drinking water electrochemical disinfection. Sci. Total. Environ. 637, 408–417 (2018).
Karkooti, A. et al. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Membr. Sci. 560, 97–107 (2018).
Su, Z., Ding, J. & Wei, G. Electrospinning: a facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. Rsc. Adv. 4, 52598–52610 (2014).
Pervez, M. N. & Stylios, G. K. Investigating the synthesis and characterization of a novel “green” H2O2-assisted, water-soluble chitosan/polyvinyl alcohol nanofiber for environmental end uses. Nanomaterials 8, 395 (2018).
Pervez, M. N. & Stylios, G. K. An experimental approach to the synthesis and optimisation of a ‘green’nanofibre. Nanomaterials 8, 383 (2018).
Pervez, M. N., Stylios, G. K., Liang, Y., Ouyang, F. & Cai, Y. Low-temperature synthesis of novel polyvinylalcohol (PVA) nanofibrous membranes for catalytic dye degradation. J. Clean. Prod. 262, 121301 (2020).
Xue, J., Wu, T., Dai, Y. & Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 119, 5298–5415 (2019).
Alias, N. H. et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 360, 1437–1446 (2019).
Cheng, C., Li, X., Yu, X., Wang, M. & Wang, X. Electrospinning: Nanofabrication and Applications. Ch. 14 (Elsevier, Norwich, 2019).
Sundaran, S. P., Reshmi, C. R., Sagitha, P., Manaf, O. & Sujith, A. Multifunctional graphene oxide loaded nanofibrous membrane for removal of dyes and coliform from water. J. Environ. Manag. 240, 494–503 (2019).
Hosseini, S. A., Vossoughi, M., Mahmoodi, N. M. & Sadrzadeh, M. Clay-based electrospun nanofibrous membranes for colored wastewater treatment. Appl. Clay. Sci. 168, 77–86 (2019).
Bjorge, D. et al. Performance assessment of electrospun nanofibers for filter applications. Desalination 249, 942–948 (2009).
Daels, N. et al. The use of electrospun flat sheet nanofibre membranes in MBR applications. Desalination 257, 170–176 (2010).
Kim, H.-C. et al. Electrospun nanofibrous PVDF–PMMA MF membrane in laboratory and pilot-scale study treating wastewater from seoul zoo. Desalination 346, 107–114 (2014).
Zhao, F. et al. Antimicrobial three dimensional woven filters containing silver nanoparticle doped nanofibers in a membrane bioreactor for wastewater treatment. Sep. Purif. Technol. 175, 130–139 (2017).
Jin, M.-Y., Liao, Y., Loh, C. H., Tan, C.-H. & Wang, R. Preparation of polydimethylsiloxane–polyvinylidene fluoride composite membranes for phenol removal in extractive membrane bioreactor. Ind. Eng. Chem. Res. 56, 3436–3445 (2017).
Liao, Y., Goh, S., Tian, M., Wang, R. & Fane, A. G. Design, development and evaluation of nanofibrous composite membranes with opposing membrane wetting properties for extractive membrane bioreactors. J. Membr. Sci. 551, 55–65 (2018).
Ren, L.-F. et al. High-performance electrospinning-phase inversion composite PDMS membrane for extractive membrane bioreactor: fabrication, characterization, optimization and application. J. Membr. Sci. 597, 117624 (2020).
Ren, L.-F. et al. Novel external extractive membrane bioreactor (EMBR) using electrospun polydimethylsiloxane/polymethyl methacrylate membrane for phenol-laden saline wastewater. Chem. Eng. J. 383, 123179 (2020).
Gee, S. & Smith, A. L. Development of electrospun nanofiber membranes for anaerobic membrane bioreactors, 16th World Conference on Anaerobic Digestion Conference AD16. (Delft, The Netherlands, 2019).
Mahmoodi, N. M. & Saffar-Dastgerdi, M. H. Zeolite nanoparticle as a superior adsorbent with high capacity: synthesis, surface modification and pollutant adsorption ability from wastewater. Microchem. J. 145, 74–83 (2019).
Nasseh, N., Taghavi, L., Barikbin, B. & Nasseri, M. A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 179, 42–54 (2018).
Ahmad, A. et al. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. Rsc. Adv. 5, 30801–30818 (2015).
Al-Hamadani, Y. A. et al. Stabilization and dispersion of carbon nanomaterials in aqueous solutions: a review. Sep. Purif. Technol. 156, 861–874 (2015).
Qu, X., Alvarez, P. J. & Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 47, 3931–3946 (2013).
Su, Y.-C., Huang, C., Pan, J. R., Hsieh, W.-P. & Chu, M.-C. Fouling mitigation by TiO2 composite membrane in membrane bioreactors. J. Environ. Eng. 138, 344–350 (2011).
Liu, L., Zhao, C. & Yang, F. TiO2 and polyvinyl alcohol (PVA) coated polyester filter in bioreactor for wastewater treatment. Water Res. 46, 1969–1978 (2012).
Hu, W., Yin, J., Deng, B. & Hu, Z. Application of nano TiO2 modified hollow fiber membranes in algal membrane bioreactors for high-density algae cultivation and wastewater polishing. Bioresour. Technol. 193, 135–141 (2015).
Tavakolmoghadam, M., Mohammadi, T., Hemmati, M. & Naeimpour, F. Surface modification of PVDF membranes by sputtered TiO2: fouling reduction potential in membrane bioreactors. Desalin. Water Treat. 57, 3328–3338 (2016).
Fonouni, M., Etemadi, H., Yegani, R. & Zarin, S. Fouling characterization of TiO2 nanoparticle embedded polypropylene membrane in oil refinery wastewater treatment using membrane bioreactor (MBR). Desalin. Water Treat. 90, 99–109 (2017).
Etemadi, H., Milad, F. & Yegani, R. Investigation of antifouling properties of polypropylene/TiO2 nanocomposite membrane under different aeration rate in membrane bioreactor system. Biotechnol. Rep. 25, e00414 (2020).
Homayoonfal, M., Mehrnia, M. R., Rahmani, S. & Mojtahedi, Y. M. Fabrication of alumina/polysulfone nanocomposite membranes with biofouling mitigation approach in membrane bioreactors. J. Ind. Eng. Chem. 22, 357–367 (2015).
Amini, M., Etemadi, H., Akbarzadeh, A. & Yegani, R. Preparation and performance evaluation of high-density polyethylene/silica nanocomposite membranes in membrane bioreactor system. Biochem. Eng. J. 127, 196–205 (2017).
Liang, S. et al. Organic fouling behavior of superhydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes functionalized with surface-tailored nanoparticles: implications for organic fouling in membrane bioreactors. J. Membr. Sci. 463, 94–101 (2014).
Li, N., Liu, L. & Yang, F. Highly conductive graphene/PANi-phytic acid modified cathodic filter membrane and its antifouling property in EMBR in neutral conditions. Desalination 338, 10–16 (2014).
Liu, L., Zhao, F., Liu, J. & Yang, F. Preparation of highly conductive cathodic membrane with graphene (oxide)/PPy and the membrane antifouling property in filtrating yeast suspensions in EMBR. J. Membr. Sci. 437, 99–107 (2013).
Alsalhy, Q. F., Al-Ani, F. H., Al-Najar, A. E. & Jabuk, S. I. A study of the effect of embedding ZnO-NPs on PVC membrane performance use in actual hospital wastewater treatment by membrane bioreactor. Chem. Eng. Process. Process. Intensif. 130, 262–274 (2018).
Etemadi, H., Yegani, R. & Seyfollahi, M. The effect of amino functionalized and polyethylene glycol grafted nanodiamond on anti-biofouling properties of cellulose acetate membrane in membrane bioreactor systems. Sep. Purif. Technol. 177, 350–362 (2017).
Tizchang, A., Jafarzadeh, Y., Yegani, R. & Khakpour, S. The effects of pristine and silanized nanodiamond on the performance of polysulfone membranes for wastewater treatment by MBR system. J. Environ. Chem. Eng. 7, 103447 (2019).
Kivi, M. A., Alinia, H., Jafarzadeh, Y. & Yegani, R. High-density polyethylene membranes embedded with carboxylated and polyethylene glycol-grafted nanodiamond to be used in membrane bioreactors. J. Appl. Polym. Sci. 136, 47914 (2019).
Pirsaheb, M. et al. Fabrication of high-performance antibiofouling ultrafiltration membranes with potential application in membrane bioreactors (MBRs) comprising polyethersulfone (PES) and polycitrate-Alumoxane (PC-A). Sep. Purif. Technol. 211, 618–627 (2019).
Mahmoudi, E., Ng, L. Y., Ang, W. L., Teow, Y. H. & Mohammad, A. W. Improving membrane bioreactor performance through the synergistic effect of silver-decorated graphene oxide in composite membranes. J. Water Process. Eng. 34, 101169 (2020).
Behboudi, A., Jafarzadeh, Y. & Yegani, R. Incorporation of silica grafted silver nanoparticles into polyvinyl chloride/polycarbonate hollow fiber membranes for pharmaceutical wastewater treatment. Chem. Eng. Res. Des. 135, 153–165 (2018).
Ahmad, J., Naeem, S., Ahmad, M., Usman, A. R. & Al-Wabel, M. I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manag. 246, 214–228 (2019).
Chen, H. et al. Enhanced adsorption of U (VI) and 241Am (III) from wastewater using Ca/Al layered double hydroxide@ carbon nanotube composites. J. Hazard. Mater. 347, 67–77 (2018).
De Volder, M. F., Tawfick, S. H., Baughman, R. H. & Hart, A. J. Carbon nanotubes: present and future commercial applications. Science 339, 535–539 (2013).
Lee, K.-J. & Park, H.-D. Effect of transmembrane pressure, linear velocity, and temperature on permeate water flux of high-density vertically aligned carbon nanotube membranes. Desalin. Water Treat. 57, 26706–26717 (2016).
Lee, B. et al. A carbon nanotube wall membrane for water treatment. Nat. Commun. 6, 7109 (2015).
Yang, Y., Qiao, S., Jin, R., Zhou, J. & Quan, X. A novel aerobic electrochemical membrane bioreactor with CNTs hollow fiber membrane by electrochemical oxidation to improve water quality and mitigate membrane fouling. Water Res. 151, 54–63 (2019).
Mulopo, J. Bleach plant effluent treatment in anaerobic membrane bioreactor (AMBR) using carbon nanotube/polysulfone nanocomposite membranes. J. Environ. Chem. Eng. 5, 4381–4387 (2017).
Khalid, A., Abdel-Karim, A., Atieh, M. A., Javed, S. & McKay, G. PEG-CNTs nanocomposite PSU membranes for wastewater treatment by membrane bioreactor. Sep. Purif. Technol. 190, 165–176 (2018).
Ling, S. et al. Design and function of biomimetic multilayer water purification membranes. Sci. Adv. 3, e1601939 (2017).
Abouzeid, R. E., Khiari, R., El-Wakil, N. & Dufresne, A. Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromolecules 20, 573–597 (2018).
Carpenter, A. W., de Lannoy, C.-F. & Wiesner, M. R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 49, 5277–5287 (2015).
Ni, X., Cheng, W., Huan, S., Wang, D. & Han, G. Electrospun cellulose nanocrystals/poly (methyl methacrylate) composite nanofibers: morphology, thermal and mechanical properties. Carbohyd. Polym. 206, 29–37 (2019).
Zhou, C., Chu, R., Wu, R. & Wu, Q. Electrospun polyethylene oxide/cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 12, 2617–2625 (2011).
He, J. et al. Diffusion and filtration properties of self-assembled gold nanocrystal membranes. Nano. Lett. 11, 2430–2435 (2011).
Goetz, L. A., Jalvo, B., Rosal, R. & Mathew, A. P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 510, 238–248 (2016).
Meng, F. et al. Fouling in membrane bioreactors: an updated review. Water Res. 114, 151–180 (2017).
Bellona, C. & Drewes, J. E. The role of membrane surface charge and solute physico-chemical properties in the rejection of organic acids by NF membranes. J. Membr. Sci. 249, 227–234 (2005).
Lin, H. et al. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 460, 110–125 (2014).
Wang, X. et al. Fabrication of PbO2 tipped Co3O4 nanowires for efficient photoelectrochemical decolorization of dye (reactive brilliant blue KN-R) wastewater. Sol. Energ. Mat. Sol. C. 191, 381–388 (2019).
Wang, S. et al. Lead-free sodium niobate nanowires with strong piezo-catalysis for dye wastewater degradation. Ceram. Int. 45, 11703–11708 (2019).
Jeevanandam, J. et al. Sustainable Nanoscale Engineering. Ch. 4 (Elsevier, Amsterdam, 2020).
Jiang, R., Wen, W. & Wu, J.-M. Titania nanowires coated PEI/P25 membranes for photocatalytic and ultrafiltration applications. New. J. Chem. 42, 3020–3027 (2018).
Zhang, X., Du, A. J., Pan, J., Wang, Y. & Sun, D. D. New Membranes and Advanced Materials for Wastewater Treatment. Ch. 14 (American Chemical Society, Washington DC, 2009).
Zhou, J. et al. Two-dimensional superstructures filled into polysulfone membranes for highly improved ultrafiltration: the case of cuprous iodide nanosheets. J. Membr. Sci. 576, 142–149 (2019).
Yang, G. et al. A novel nanocomposite membrane combining BN nanosheets and GO for effective removal of antibiotic in water. Nanomaterials 9, 386 (2019).
Kashefi, S., Borghei, S. M. & Mahmoodi, N. M. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 276, 153–162 (2019).
Zhou, T., Ma, L., Gan, M., Wang, H. & Hao, C. Sandwich-structured hybrids: a facile electrostatic self-assembly of exfoliated titania nanosheets and polyaniline nanoparticles and its high visible-light photocatalytic performance. J. Phys. Chem. Solids 125, 123–130 (2019).
Liang, X. et al. Zwitterionic functionalized MoS2 nanosheets for a novel composite membrane with effective salt/dye separation performance. J. Membr. Sci. 573, 270–279 (2019).
Xiao, G. et al. Superior adsorption performance of graphitic carbon nitride nanosheets for both cationic and anionic heavy metals from wastewater. Chin. J. Chem. Eng. 27, 305–313 (2019).
Ghalamchi, L., Aber, S., Vatanpour, V. & Kian, M. A novel antibacterial mixed matrixed PES membrane fabricated from embedding aminated Ag3PO4/g-C3N4 nanocomposite for use in the membrane bioreactor. J. Ind. Eng. Chem. 70, 412–426 (2019).
Zinadini, S. et al. Preparation and characterization of antifouling graphene oxide/polyethersulfone ultrafiltration membrane: application in MBR for dairy wastewater treatment. J. Water Process. Eng. 7, 280–294 (2015).
Beygmohammdi, F., Kazerouni, H. N., Jafarzadeh, Y., Hazrati, H. & Yegani, R. Preparation and characterization of PVDF/PVP-GO membranes to be used in MBR system. Chem. Eng. Res. Des. 154, 232–240 (2020).
Ghaffar, A., Zhang, L., Zhu, X. & Chen, B. Porous PVdF/GO nanofibrous membranes for selective separation and recycling of charged organic dyes from water. Environ. Sci. Technol. 52, 4265–4274 (2018).
Wu, W. et al. Enhanced MPBR with polyvinylpyrrolidone-graphene oxide/PVDF hollow fiber membrane for efficient ammonia nitrogen wastewater treatment and high-density Chlorella cultivation. Chem. Eng. J. 379, 122368 (2020).
Field, R. Membrane Technology: Membranes for Water Treatment. Ch. 1 (Wiley-VCH, Weinheim, 2010).
Mutamim, N. S. A., Noor, Z. Z., Hassan, M. A. A., Yuniarto, A. & Olsson, G. Membrane bioreactor: applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 225, 109–119 (2013).
Cho, B. D. & Fane, A. G. Fouling transients in nominally sub-critical flux operation of a membrane bioreactor. J. Membr. Sci. 209, 391–403 (2002).
Lin, H. et al. A review on anaerobic membrane bioreactors: applications, membrane fouling and future perspectives. Desalination 314, 169–188 (2013).
Lv, D. et al. Ecofriendly electrospun membranes loaded with visible-light-responding nanoparticles for multifunctional usages: highly efficient air filtration, dye scavenging, and bactericidal activity. Acs. Appl. Mater. Inter. 11, 12880–12889 (2019).
Ren, Y. et al. Stereocomplex crystallites-based eco-friendly nanofiber membranes for removal of Cr (VI) and antibacterial effect. Acs. Sustain. Chem. Eng. 7, 16072–16083 (2019).
Kamali, M., Persson, K. M., Costa, M. E. & Capela, I. Sustainability criteria for assessing nanotechnology applicability in industrial wastewater treatment: current status and future outlook. Environ. Int. 125, 261–276 (2019).
Wu, M. et al. Fabrication of composite nanofiltration membrane by incorporating attapulgite nanorods during interfacial polymerization for high water flux and antifouling property. J. Membr. Sci. 544, 79–87 (2017).
Arkas, M., Allabashi, R., Tsiourvas, D., Mattausch, E.-M. & Perfler, R. Organic/inorganic hybrid filters based on dendritic and cyclodextrin “nanosponges” for the removal of organic pollutants from water. Environ. Sci. Technol. 40, 2771–2777 (2006).
Zhao, G., Hu, R., Li, J. & Zhu, H. Graphene oxide quantum dots embedded polysulfone membranes with enhanced hydrophilicity, permeability and antifouling performance. Sci. China Mater. 62, 1177–1187 (2019).
Fatarella, E., Iversen, V., Grinwis, S. & Paulussen, S. Textile material for membrane filtration, AMADEUS final report (2009).
Bojnourd, F. M. & Pakizeh, M. Preparation and characterization of a nanoclay/PVA/PSf nanocomposite membrane for removal of pharmaceuticals from water. Appl. Clay. Sci. 162, 326–338 (2018).
Ahmad, T., Guria, C. & Mandal, A. Optimal synthesis and operation of low-cost polyvinyl chloride/bentonite ultrafiltration membranes for the purification of oilfield produced water. J. Membr. Sci. 564, 859–877 (2018).
Bohdziewicz, J., Neczaj, E. & Kwarciak, A. Landfill leachate treatment by means of anaerobic membrane bioreactor. Desalination 221, 559–565 (2008).
Tarnacki, K., Lyko, S., Wintgens, T., Melin, T. & Natau, F. Impact of extra-cellular polymeric substances on the filterability of activated sludge in membrane bioreactors for landfill leachate treatment. Desalination 179, 181–190 (2005).
Dabaghian, Z., Peyravi, M., Jahanshahi, M. & Rad, A. S. Potential of advanced nano-structured membranes for landfill leachate treatment: a review. ChemBioEng. Rev. 5, 119–138 (2018).
Casals, E. et al. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 10, 2801–2808 (2014).
Zhang, L. et al. Evaluating the influences of ZnO engineering nanomaterials on VFA accumulation in sludge anaerobic digestion. Biochem. Eng. J. 125, 206–211 (2017).
Baniamerian, H. et al. Application of nano-structured materials in anaerobic digestion: current status and perspectives. Chemosphere 229, 188–199 (2019).
Atasoy, M., Owusu-Agyeman, I., Plaza, E. & Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: current status and future challenges. Bioresour. Technol. 268, 773–786 (2018).
Parchami, M., Wainaina, S., Mahboubi, A., I’Ons, D. & Taherzadeh, M. J. MBR-assisted VFAs production from excess sewage sludge and food waste slurry for sustainable wastewater treatment. Appl. Sci. 10, 2921 (2020).
Paudel, S. et al. Anaerobic hydrogen fermentation and membrane bioreactor (MBR) for decentralized sanitation and reuse-organic removal and resource recovery. Environ. Eng. Res. 19, 387–393 (2014).
Nguyen, A. Q. et al. Derivation of volatile fatty acid from crop residues digestion using a rumen membrane bioreactor: a feasibility study. Bioresour. Technol. 312, 123571 (2020).
Yan, T. et al. A critical review on membrane hybrid system for nutrient recovery from wastewater. Chem. Eng. J. 348, 143–156 (2018).
Song, X. et al. Resource recovery from wastewater by anaerobic membrane bioreactors: opportunities and challenges. Bioresour. Technol. 270, 669–677 (2018).
Valsami-Jones, E. & Lynch, I. How safe are nanomaterials? Science 350, 388–389 (2015).
Wen, Y., Yuan, J., Ma, X., Wang, S. & Liu, Y. Polymeric nanocomposite membranes for water treatment: a review. Environ. Chem. Lett. 17, 1539–1551 (2019).
Goh, P. S., Ismail, A. F. & Hilal, N. Nano-enabled membranes technology: sustainable and revolutionary solutions for membrane desalination? Desalination 380, 100–104 (2016).
Westerhoff, P. et al. Low risk posed by engineered and incidental nanoparticles in drinking water. Nat. Nanotechnol. 13, 661–669 (2018).
Kellenberger, C. R. et al. Roll-to-roll preparation of mesoporous membranes by nanoparticle template removal. Ind. Eng. Chem. Res. 53, 9214–9220 (2014).
Liu, Y. et al. A facile and scalable fabrication procedure for thin-film composite membranes: Integration of phase inversion and interfacial polymerization. Environ. Sci. Technol. 54, 1946–1954 (2020).
Lalia, B. S., Kochkodan, V., Hashaikeh, R. & Hilal, N. A review on membrane fabrication: structure, properties and performance relationship. Desalination 326, 77–95 (2013).
Bhardwaj, N. & Kundu, S. C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 28, 325–347 (2010).
Singh, M. et al. Additive manufacturing of mechanically isotropic thin films and membranes via microextrusion 3D printing of polymer solutions. Acs. Appl. Mater. Inter. 11, 6652–6661 (2019).
Murthy, G. S. Bioenergy: Principles and Applications. Ch. 27 (Wiley Blackwell, Hoboken, 2016).
Roccaro, P. & Vagliasindi, F. G. A. Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability. Ch. 67 (Springer, Cham, 2020).
Aydiner, C. et al. Techno-economic viability of innovative membrane systems in water and mass recovery from dairy wastewater. J. Membr. Sci. 458, 66–75 (2014).
Bertanza, G. et al. A comparison between two full-scale MBR and CAS municipal wastewater treatment plants: techno-economic-environmental assessment. Environ. Sci. Pollut. R. 24, 17383–17393 (2017).
Acknowledgements
We would like to express their sincere gratitude to the support from (i) Sanitary Environmental Engineering Division (SEED) and grants (FARB projects) from the University of Salerno coordinated by prof. V. Naddeo; (ii) Inter-University Consortium of Relevant Hazardous (Consorzio inter-Universitario per la previsione e la prevenzionedei Grandi Rischi, C.U.G.RI.); (iii) grant INT/Italy/P-17/2016 (SP) from Department of Science and Technology, Ministry of Science and Technology, Government of India; (iv) grant (No. 2019R1H1A2080148) from the National Research Foundation of Korea, funded by the Korean government; and (v) the Center for Membranes and Advanced Water technology (CMAT) at Khalifa University of Science and Technology (Abu Dhabi, UAE).
Author information
Authors and Affiliations
Contributions
M.N.P., T.Z., V.N.—concept and original draft, M.B.—reviewing & editing, S.W.H.—reviewing & editing, K.H.C.—reviewing & editing, Y.Z.— reviewing & editing, Y.C.—reviewing & editing, V.B.—reviewing & editing, T.Z.—reviewing & editing, V.N.—reviewing, editing, & supervision. Finally, all authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pervez, M.N., Balakrishnan, M., Hasan, S.W. et al. A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment. npj Clean Water 3, 43 (2020). https://doi.org/10.1038/s41545-020-00090-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-020-00090-2
This article is cited by
-
Polyamidoamine-based graphene oxide nanoparticles as adsorbent for mitigation of fouling in electrochemical membrane bioreactor
International Journal of Environmental Science and Technology (2024)
-
Tuning the surface functionality of polyethylene glycol-modified graphene oxide/chitosan composite for efficient removal of dye
Scientific Reports (2023)
-
Effect of packing media type of moving and fixed on performance and membrane fouling in biofilm-based MBR processes
Biomass Conversion and Biorefinery (2023)
-
A Comprehensive Review on Application of Lignocellulose Derived Nanomaterial in Heavy Metals Removal from Wastewater
Chemistry Africa (2023)
-
Prediction of the Diameter of Biodegradable Electrospun Nanofiber Membranes: An Integrated Framework of Taguchi Design and Machine Learning
Journal of Polymers and the Environment (2023)