Abstract
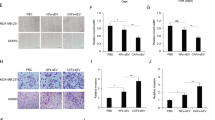
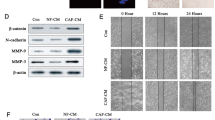
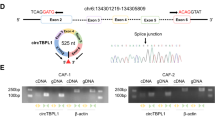
Cancer-associated fibroblasts (CAFs) play supporting roles in tumor progression by releasing microvesicles that transmit oncogenic cargoes. Indeed, extracellular vesicles (EVs) have emerged as important vehicles to deliver proteins, messenger RNAs (mRNAs), and microRNAs (miRs) between cells. In this study, we aimed to outline the role and function of CAFs-derived EVs carrying miR-1-3p in breast cancer. We first experimentally determined downregulated miR-1-3p in breast cancer tissues. EVs were isolated from CAFs extracted from breast cancer tissues, which showed downregulated miR-1-3p expression relative to EVs derived from normal fibroblasts (NFs). In a co-culture system, miR-1-3p cargo was transported into breast cancer cells via CAF-derived EVs. In gain-of-function experiments, the elevation of miR-1-3p in breast cancer cells inhibited cell viability, invasion, migration, and epithelial-to-mesenchymal transition, and suppressed tumor formation and metastasis. Furthermore, EVs derived from CAFs transfected with miR-1-3p mimic were more effective in transferring miR-1-3p to suppress cancer progression and metastasis. Krüppel-like zinc-finger protein Gli-similar 1 (GLIS1) was predicted to be a putative target of miR-1-3p, which was subsequently confirmed by dual-luciferase reporter assay. We then demonstrated that overexpression of GLIS1 neutralized the effects of miR-1-3 on the development of breast cancer in vitro. These findings shed light on the underlying mechanism by which CAFs-derived EVs carrying miR-1-3p mediate the progression and metastasis of breast cancer, and highlight the potential of miR-1-3p shuttled by CAFs-derived EVs serving as a therapeutic target for breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated/analyzed during this study are available.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65:221–38.
Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19:1450–64.
Wei SC, Yang J. Forcing through tumor metastasis: the interplay between tissue rigidity and epithelial-mesenchymal transition. Trends Cell Biol. 2016;26:111–20.
Johansson HJ, Socciarelli F, Vacanti NM, Haugen MH, Zhu Y, Siavelis I, et al. Breast cancer quantitative proteome and proteogenomic landscape. Nat Commun. 2019;10:1600.
Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer-the emerging science of cellular ‘debris’. Semin Immunopathol. 2011;33:455–67.
Zha QB, Yao YF, Ren ZJ, Li XJ, Tang JH. Extracellular vesicles: an overview of biogenesis, function, and role in breast cancer. Tumour Biol. 2017;39:1010428317691182.
Nassar FJ, Nasr R, Talhouk R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharm Ther. 2017;172:34–49.
Rodriguez-Martinez A, de Miguel-Perez D, Ortega FG, Garcia-Puche JL, Robles-Fernandez I, Exposito J, et al. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019;21:21.
Li XJ, Ren ZJ, Tang JH, Yu Q. Exosomal microRNA MiR-1246 promotes cell proliferation, invasion and drug resistance by targeting CCNG2 in breast cancer. Cell Physiol Biochem. 2017;44:1741–8.
Wei Y, Lai X, Yu S, Chen S, Ma Y, Zhang Y, et al. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res Treat. 2014;147:423–31.
Ke J, Zhang BH, Li YY, Zhong M, Ma W, Xue H, et al. MiR-1-3p suppresses cell proliferation and invasion and targets STC2 in gastric cancer. Eur Rev Med Pharm Sci. 2019;23:8870–7.
Li SM, Wu HL, Yu X, Tang K, Wang SG, Ye ZQ, et al. The putative tumour suppressor miR-1-3p modulates prostate cancer cell aggressiveness by repressing E2F5 and PFTK1. J Exp Clin Cancer Res. 2018;37:219.
Gao L, Yan P, Guo FF, Liu HJ, Zhao ZF. MiR-1-3p inhibits cell proliferation and invasion by regulating BDNF-TrkB signaling pathway in bladder cancer. Neoplasma. 2018;65:89–96.
Sun D, Zhong J, Wei W, Chen X, Liu J, Hu Z. Identification of microRNA expression in sentinel lymph nodes from patients with breast cancer via RNA sequencing for diagnostic accuracy. J Gene Med. 2019;21:e3075.
Gerard D, Schmidt F, Ginolhac A, Schmitz M, Halder R, Ebert P, et al. Temporal enhancer profiling of parallel lineages identifies AHR and GLIS1 as regulators of mesenchymal multipotency. Nucleic Acids Res. 2019;47:1141–63.
Vadnais C, Shooshtarizadeh P, Rajadurai CV, Lesurf R, Hulea L, Davoudi S, et al. Autocrine activation of the Wnt/beta-catenin pathway by CUX1 and GLIS1 in breast cancers. Biol Open. 2014;3:937–46.
Zhao L, Sun Y, Hou Y, Peng Q, Wang L, Luo H, et al. MiRNA expression analysis of cancer-associated fibroblasts and normal fibroblasts in breast cancer. Int J Biochem Cell Biol. 2012;44:2051–9.
Kuzet SE, Gaggioli C. Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res. 2016;365:607–19.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72.
Yang WW, Yang LQ, Zhao F, Chen CW, Xu LH, Fu J, et al. Epiregulin promotes lung metastasis of salivary adenoid cystic carcinoma. Theranostics. 2017;7:3700–14.
Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M, et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. 2017;8:19592–608.
Sharif S, Ghahremani MH, Soleimani M. Delivery of exogenous miR-124 to glioblastoma multiform cells by Wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev Rep. 2018;14:236–46.
Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554:378–81.
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–8.
Zhang J, Wang L, Mao S, Liu M, Zhang W, Zhang Z, et al. miR-1-3p contributes to cell proliferation and invasion by targeting glutaminase in bladder cancer cells. Cell Physiol Biochem. 2018;51:513–27.
Liu J, Huang Y, Cheng Q, Wang J, Zuo J, Liang Y, et al. miR-1-3p suppresses the epithelial-mesenchymal transition property in renal cell cancer by downregulating fibronectin 1. Cancer Manag Res. 2019;11:5573–87.
Pohlodek K, Bozikova S, Meciarova I, Mucha V, Bartova M, Ondrias F. Prediction of additional lymph node involvement in breast cancer patients with positive sentinel lymph nodes. Neoplasma. 2016;63:427–34.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45:229–36.
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16:148.
Jolly MK, Boareto M, Huang B, Jia D, Lu M, Ben-Jacob E, et al. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol. 2015;5:155.
Chaw SY, Abdul Majeed A, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48:997–1006.
Liu CC, Cai DL, Sun F, Wu ZH, Yue B, Zhao SL, et al. FERMT1 mediates epithelial-mesenchymal transition to promote colon cancer metastasis via modulation of beta-catenin transcriptional activity. Oncogene. 2017;36:1779–92.
Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005
Shang A, Yang M, Shen F, Wang J, Wei J, Wang W, et al. MiR-1-3p suppresses the proliferation, invasion and migration of bladder cancer cells by up-regulating SFRP1 expression. Cell Physiol Biochem. 2017;41:1179–88.
Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, Kodanaka I, Ichisaka T, et al. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225–9.
Acknowledgements
The authors sincerely appreciate all members participated in this work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81760482, 82060479), Natural Science Foundation of Ningxia (2020AAC03417, 2018AAC03165) and Changzhou Sci & Tech Program (CJ20200068).
Author information
Authors and Affiliations
Contributions
XZM and JPL wrote the paper; ST, HL, and XZM designed the research; YFM, JLH, and YLG performed the research; ST, HL, and XZM analyzed the data; all authors contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tao, S., Li, H., Ma, X. et al. Elevating microRNA-1-3p shuttled by cancer-associated fibroblasts-derived extracellular vesicles suppresses breast cancer progression and metastasis by inhibiting GLIS1. Cancer Gene Ther 28, 634–648 (2021). https://doi.org/10.1038/s41417-020-00244-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00244-x
This article is cited by
-
Crosstalk and plasticity driving between cancer-associated fibroblasts and tumor microenvironment: significance of breast cancer metastasis
Journal of Translational Medicine (2023)
-
The role of non-coding RNAs in extracellular vesicles in breast cancer and their diagnostic implications
Oncogene (2023)
-
Effect of GLIS1 on the Lymph Node Metastasis of Cervical Squamous Carcinoma Based on Transcriptome Analysis
Reproductive Sciences (2023)
-
Intercellular crosstalk between cancer cells and cancer-associated fibroblasts via extracellular vesicles
Cancer Cell International (2022)
-
Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer
Signal Transduction and Targeted Therapy (2021)