Abstract
Purpose
Hyaluronan (HA) based biomaterials are widely used as tissue scaffolds, drug formulations, as well as targeting ligands and imaging probes for diagnosis and drug delivery. However, because of the presence of abundant endogenous HA presented in various tissues in vivo, the pharmacokinetic behavior and biodistribution patterns of exogenously administered HAs have not been well characterized.
Methods
The HA backbone was modified with Diethylenetriamine (DTPA) to enable the chelation of gadolinium (Gd) and aluminum (Al) ions. Series of PET and MR imaging were taken after the injection of HA-DTPA-Gd and HA-DTPA-Al18F while using18F-FDG and Magnevist(DTPA-Gd) as controls. The Tomographic images were analyzed and quantified to reveal the distribution and locations of HA in tumor-bearing mice.
Results
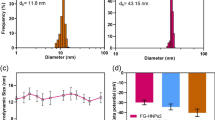
The labeled HAs had good stability in plasma. They retained binding affinity towards CD44s on tumor cell surface. The injected HAs distributed widely in various organs, but were found to be cleared quickly except inside tumor tissues where the signals were higher and persisted longer.
Conclusion
Medical imaging tools, including MR and PET, can be highly valuable for examining biomaterial distribution non-invasively. The HA tumor accumulation properties may be explored for the development of active targeting drug carriers and molecular probes.







Similar content being viewed by others
Abbreviations
- CCK-8:
-
Cell counting Kit-8
- DTPA:
-
Diethylenetriamine pentoacetic acid
- FBS:
-
Fetal bovine serum
- FOV:
-
Fields of view
- HA:
-
Hyaluronan
- HEC:
-
Hepato-enteric circulation
- PET/CT:
-
Positron emission tomography/computed tomography
- PET/MR:
-
Positron emission tomography/magnetic resonance
- MRI:
-
Magnetic resonance imaging
- NMR:
-
Nuclear magnetic resonance
- RES:
-
Reticuloendothelial System
- ROI:
-
Regions of interest
- SNR:
-
Signal-to-noise ratio
- SPECT:
-
Single-photon emission computed tomography
- SUV:
-
Standardized uptake value
References
Slomiany MG, Toole BP. CHAPTER 2 - Hyaluronan–CD44 interactions and chemoresistance in cancer cells. In: Stern R, editor. Hyaluronan in cancer biology. San Diego: Academic Press; 2009. p. 19–35.
Fraser JRE, Laurent TC. Turnover and Metabolism of Hyaluronan. In. Ciba Foundation Symposium 143 - The Biology of Hyaluronan: John Wiley & Sons, Ltd.; 2007. p. 41–59.
Schanté CE, Zuber G, Herlin C, Vandamme TF. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr Polym. 2011;85(3):469–89.
Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27–33.
Crescenzi V, Francescangeli A, Renier D, Bellini D. New cross-linked and sulfated derivatives of partially deacetylated hyaluronan: synthesis and preliminary characterization. Biopolymers. 2002;64(2):86–94.
Pulakkat S, Balaji SA, Rangarajan A, Raichur AM. Surface engineered protein nanoparticles with hyaluronic acid based multilayers for targeted delivery of anticancer agents. ACS Appl Mater Interfaces. 2016;8(36):23437–49.
Goodarzi N, Ghahremani MH, Amini M, Atyabi F, Ostad SN, Shabani Ravari N, et al. CD44-targeted docetaxel conjugate for cancer cells and cancer stem-like cells: a novel hyaluronic acid-based drug delivery system. Chem Biol Drug Des. 2014;83(6):741–52.
Maiolino S, Moret F, Conte C, Fraix A, Tirino P, Ungaro F, et al. Hyaluronan-decorated polymer nanoparticles targeting the CD44 receptor for the combined photo/chemo-therapy of cancer. Nanoscale. 2015;7(13):5643–53.
Naor D. Editorial: interaction between hyaluronic acid and its receptors (CD44, RHAMM) regulates the activity of inflammation and cancer. Front Immunol. 2016;7(39).
Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between Hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol. 2015;6:201.
Chanmee T, Ontong P, Kimata K, Itano N. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front Oncol. 2015;5:180.
Underhill CB. The interaction of hyaluronate with the cell surface: the hyaluronate receptor and the core protein. In. Ciba Foundation Symposium 143 - The Biology of Hyaluronan: John Wiley & Sons, Ltd.; 2007. p. 87–106.
Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4(9):1033–43.
Song JM, Im J, Nho RS, Han YH, Upadhyaya P, Kassie F. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol Carcinog. 2019;58(3):321–33.
Sun SJ, Wu CC, Sheu GT, Chang HY, Chen MY, Lin YY, et al. Integrin beta3 and CD44 levels determine the effects of the OPN-a splicing variant on lung cancer cell growth. Oncotarget. 2016;7(34):55572–84.
van der Voort R, Manten-Horst E, Smit L, Ostermann E, van den Berg F, Pals ST. Binding of cell-surface expressed CD44 to hyaluronate is dependent on splicing and cell type. Biochem Biophys Res Commun. 1995;214(1):137–44.
Vugts DJ, Heuveling DA, Stigter-van Walsum M, Weigand S, Bergstrom M, van Dongen GA, et al. Preclinical evaluation of 89Zr-labeled anti-CD44 monoclonal antibody RG7356 in mice and cynomolgus monkeys: prelude to phase 1 clinical studies. MAbs. 2014;6(2):567–75.
Isacke CM, Yarwood H. The hyaluronan receptor, CD44. Int J Biochem Cell Biol. 2002;34(7):718–21.
Platt VM, Szoka FC Jr. Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5(4):474–86.
Battistini FD, Flores-Martin J, Olivera ME, Genti-Raimondi S, Manzo RH. Hyaluronan as drug carrier. The in vitro efficacy and selectivity of hyaluronan-doxorubicin complexes to affect the viability of overexpressing CD44 receptor cells. Eur J Pharm Sci. 2014;65:122–9.
Cho HJ, Yoon HY, Koo H, Ko SH, Shim JS, Cho JH, et al. Hyaluronic acid-ceramide-based optical/MR dual imaging nanoprobe for cancer diagnosis. J Control Release. 2012;162(1):111–8.
Lee J-Y, Chung S-J, Cho H-J, Kim D-D. Iodinated hyaluronic acid oligomer-based nanoassemblies for tumor-targeted drug delivery and cancer imaging. Biomaterials. 2016;85(Supplement C):218–31.
Wang L, Draz MS, Wang W, Liao G, Xu Y. The quality of in vivo upconversion fluorescence signals inside different anatomic structures. J Biomed Nanotechnol. 2015;11(2):325–33.
Czernin J. Molecular imaging and therapy with a purpose: a renaissance of nuclear medicine. J Nucl Med. 2017;58(1):21A–2A.
Pichler BJ, Kolb A, Nägele T, Schlemmer H-P. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med. 2010;51(3):333–6.
Blanchet EM, Millo C, Martucci V, Maass-Moreno R, Bluemke DA, Pacak K. Integrated whole-body PET/MRI with 18F-FDG, 18F-FDOPA, and 18F-FDA in paragangliomas in comparison with PET/CT: NIH first clinical experience with a single-injection, dual-modality imaging protocol. Clin Nucl Med. 2014;39(3):243–50.
Spick C, Herrmann K, Czernin J. 18F-FDG PET/CT and PET/MRI perform equally well in Cancer: evidence from studies on more than 2,300 patients. J Nucl Med. 2016;57(3):420–30.
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99(9):2293–352.
Shiftan L, Neeman M. Kinetic analysis of hyaluronidase activity using a bioactive MRI contrast agent. Contrast Media Mol Imaging. 2006;1(3):106–12.
Yim H, Yang SG, Jeon YS, Park IS, Kim M, Lee DH, et al. The performance of gadolinium diethylene triamine pentaacetate-pullulan hepatocyte-specific T1 contrast agent for MRI. Biomaterials. 2011;32(22):5187–94.
Rui M, Tang H, Li Y, Wei X, Xu Y. Recombinant high density lipoprotein nanoparticles for target-specific delivery of siRNA. Pharm Res. 2013;30(5):1203–14.
Rui M, Guo W, Ding Q, Wei X, Xu J, Xu Y. Recombinant high-density lipoprotein nanoparticles containing gadolinium-labeled cholesterol for morphologic and functional magnetic resonance imaging of the liver. Int J Nanomedicine. 2012;7:3751–68.
McBride WJ, Sharkey RM, Karacay H, D'Souza CA, Rossi EA, Laverman P, et al. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50(6):991–8.
Li Y, Zhang D, Shi Y, Guo Z, Wu X, Ren J-L, et al. Syntheses and preliminary evaluation of [18F]AlF-NOTA-G-TMTP1 for PET imaging of high aggressive hepatocellular carcinoma. Contrast Media Mol Imaging. 2016;11(4):262–71.
Ugi I, Domling A, Werner B. Since 1995 the new chemistry of multicomponent reactions and their libraries, including their heterocyclic chemistry. J Heterocyclic Chem. 2000;37(3):647–58.
Hua Q, Knudson CB, Knudson W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J Cell Sci. 1993;106(Pt 1):365–75.
Embry JJ, Knudson W. G1 domain of aggrecan cointernalizes with hyaluronan via a CD44-mediated mechanism in bovine articular chondrocytes. Arthritis Rheum. 2003;48(12):3431–41.
Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17(1):62–70.
Lin J, Wu H, Wang Y, Lin J, Chen Q, Zhu X. Preparation and ocular pharmacokinetics of hyaluronan acid-modified mucoadhesive liposomes. Drug Deliv. 2016;23(4):1144–51.
Jeong YI, Kim ST, Jin SG, Ryu HH, Jin YH, Jung TY, et al. Cisplatin-incorporated hyaluronic acid nanoparticles based on ion-complex formation. J Pharm Sci. 2008;97(3):1268–76.
Bouziotis P, Psimadas D, Tsotakos T, Stamopoulos D, Tsoukalas C. Radiolabeled iron oxide nanoparticles as dual-modality SPECT/MRI and PET/MRI agents. Curr Top Med Chem. 2012;12(23):2694–702.
Hnatowich DJ. Label stability in serum of four radionuclides on DTPA-coupled antibodies—an evaluation. Int J Rad Appl Instrum B. 1986;13(4):353–8.
Treglia G, Sadeghi R, Del Sole A, Giovanella L. Diagnostic performance of PET/CT with tracers other than F-18-FDG in oncology: an evidence-based review. Clin Transl Oncol. 2014;16(9):770–5.
Hai W, Wu X, Shi S, Yang Y, Yang Z, Li B, et al. The effects of season change and fasting on Brown adipose tissue FDG-PET in mice. Biochem Biophys Res Commun. 2020;529(2):398–403.
Babasola O, Rees-Milton KJ, Bebe S, Wang J, Anastassiades TP. Chemically modified N-acylated hyaluronan fragments modulate proinflammatory cytokine production by stimulated human macrophages. J Biol Chem. 2014;289(36):24779–91.
Yamawaki H, Hirohata S, Miyoshi T, Takahashi K, Ogawa H, Shinohata R, et al. Hyaluronan receptors involved in cytokine induction in monocytes. Glycobiology. 2009;19(1):83–92.
Rossin R, Robillard MS. Pretargeted imaging using bioorthogonal chemistry in mice. Curr Opin Chem Biol. 2014;21:161–9.
Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, et al. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J Nucl Med. 2013;54(8):1389–96.
Goldenberg DM, Chang CH, Rossi EA, JW MB, Sharkey RM. Pretargeted molecular imaging and radioimmunotherapy. Theranostics. 2012;2(5):523–40.
ACKNOWLEDGMENTS AND DISCLOSURES
We would like to thank Shanghai Atom Kexing Pharmaceuticals Co., Ltd., for supplying the 18F fluorine. This study was supported by the National Natural Science Foundation of China (No. 81571787 and 81690262), Ruijin Youth NSFC Cultivation Fund (No. 2019QNPY02019), and the Medical-Engineering Joint Fund of Shanghai Jiao Tong University (No. YG2015MS54).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hai, W., Bao, X., Sun, K. et al. The Labeling, Visualization, and Quantification of Hyaluronan Distribution in Tumor-Bearing Mouse Using PET and MR Imaging. Pharm Res 37, 237 (2020). https://doi.org/10.1007/s11095-020-02957-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02957-y