Abstract
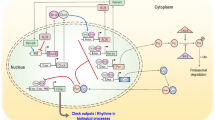
The suprachiasmatic nucleus (SCN) that acts as the primary circadian pacemaker in mammals is responsible for orchestrating multiple circadian rhythms in every organism. A network structure in the SCN composed of multiple types of neurons orchestrates the circadian rhythms. Despite speculations regarding the working of the clock, the molecular mechanisms governing it is far from clear. The molecular mechanism seems to be woven around the genes present and their linking with the neuromodulators. With the advancement in knowledge regarding the role of neuromodulators in the workings of the clock, especially that of Arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP), the entire picture of the mechanisms involved and therefore the importance of these neuromodulators has changed considerably. AVP seems to be very important for the functioning of the clock and its role has been well established based on the evidence available at present. Enormous research is going on to study the role of AVP and new roles are likely to be assigned to AVP in the execution of function in the SCN. Of late, there have been reports indicating linkage of AVP with jet lag in a positive way, suggesting vasopressin signalling as a possible remedy for ill effects and their improvement. Studies also show circadian rhythm disturbances in mood disorders and the same is related to AVP levels in the SCN. Various findings are thus in accordance with strong suggestions for a critical role for AVP in SCN function.
Similar content being viewed by others
Abbreviations
- AVP:
-
arginine vasopressin
- AVP-ir:
-
AVP immunoreactive
- BMAL 1:
-
brain-muscle-Arnt-like-protein 1
- CCGs:
-
clock controlled genes
- cry1 :
-
cryptochrome 1
- cry2 :
-
cryptochrome 2
- CSF:
-
cerebrospinal fluid
- DM:
-
dorsomedial
- ENK:
-
encephalin
- GABA:
-
gamma amino butyric acid
- GRP:
-
gastrin releasing peptide
- m tim:
-
m timeless
- mper:
-
m period
- NMS:
-
neuromedin
- PACAP:
-
pituitary adenylate cyclase-activating polypeptide
- per2:
-
period2
- per1:
-
period 1
- pk2:
-
prokineticin 2
- RGCs:
-
retinal ganglion cells
- RHT:
-
retinohypothalamic tract
- SCN:
-
suprachiasmatic nucleus
- TTFL:
-
transcription-and translational feedback loops
- UBR4:
-
ubiquitin protein ligase E3 component N-recognin
- VIP:
-
vasoactive intestinal polypeptide
- VIP-ir:
-
VIP immunoreactive
- VIP-KO:
-
VIP-deficient
- VL:
-
ventrolateral
- WT:
-
wild type
References
Akiyama M, Kouzu, Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, et al. 1999 Inhibition of light- or glutamate-induced mper1expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J. Neurosci. 19 1115–1121
Albers H, Ferris C, Leeman S and Goldman B 1984 Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science 223 833–835
Albers HE, Walton JC, Gamble KL, Mcneill JK and Hummer DL 2017 The dynamics of GABA signaling: revelations from the circadian pacemaker in the suprachiasmatic nucleus. Front. Neuroendocrinol. 44 35–82
Astiz M, Heyde L and Oster H 2019 Mechanisms of communication in the mammalian circadian timing system. Int. J. Mol. Sci. 20 E343
Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L and De Pietri Tonelli D 2017 Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat. Commun. 8 14336
Bedont JL, Rohr KE, Bathini A, Hattar S, Blackshaw S, Sehgal, A and Evans JA 2018 Asymmetric vasopressin signaling spatially organizes the master circadian clock. J. Comp. Neurol. 526 2048–2067
Brancaccio M, Enoki R, Mazuski CN, Jones J, Evans JA and Azzi A 2014 Network-mediated encoding of circadian time: the suprachiasmatic nucleus (SCN) from genes to neurons to circuits, and back. J. Neurosci. 34 15192–15199
Brown MH and Nunez AA 1989 Vasopressin-deficient rats show a reduced amplitude of the circadian sleep rhythm. Physiol. Behav. 46 759–762
Buhr ED and Takahashi JS 2013 Molecular components of the Mammalian circadian clock. Handb. Exp. Pharmacol. 15 3–27
Bunger MK Wilsbacher LD Moran SM Clendenin C Radcliffe LA Hogenesch JB et al. 2000 Mop3 Is an essential component of the master circadian pacemaker in mammals. Cell 103 1009–1017
Burkeen JF, Womac AD, Earnest DJ and Zoran MJ 2011 Mitochondrial calcium signaling mediates rhythmic extracellular atp accumulation in suprachiasmatic nucleus astrocytes. J. Neurosci. 31 8432–8440
Castel M, Feinstein N, Cohen S and Harari N 1990 Vasopressinergic innervation of the mouse suprachiasmatic nucleus: An immuno-electron microscopic analysis. J. Comp. Neurol. 298 172–187
Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Zhou QY et al. 2002 Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417 405–410.
Dardente H and Cermakian N 2007 Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol. Int. 24 195–213
Decoursey PJ and Buggy J 1989 Circadian rhythmicity after neural transplant to hamster third ventricle: specificity of suprachiasmatic nuclei. Brain Res. 500 263–275
Dunlap JC 1999). Molecular bases for circadian clocks. Cell 96 271–290
Edwards MD, Brancaccio M, Chesham JE, Maywood ES and Hastings MH 2016 Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc. Natl. Acad. Sci.USA 113 2732–2737
Evans JA, Leise TL, Castanon-Cervantes O and Davidson AJ 2013 Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron 80 973–983
Evans JA 2016 Collective timekeeping among cells of the master circadian clock. J. Endocrinol. 230 R27–R49
Field MD, Maywood ES, O′Brien JA, Weaver DR, Reppert SM and Hastings M 2000 Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanism. Neuron 25 437–447
Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR and Maier SF 2015 Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav. Immun. 45 171–179
Francl JM, Kaur G and Glass JD 2010 Regulation of vasoactive intestinal polypeptide release in the suprachiasmatic nucleus circadian clock. Neuroreport 21 1055–1059
Frenkel L, Muraro NI, González ANB, Marcora MS, Bernabó G, Hermann-Luibl, et al. 2017 Organization of circadian behavior relies on glycinergic transmission. Cell Rep. 19 72–85
Granados-Fuentes D and Herzog, ED 2013 The clock shop: Coupled circadian oscillators. Expt. Neurol. 243 21–27
Groblewski TA, Nunez AA and Gold RM 1981 Circadian rhythms in vasopressin deficient rats. Brain Res. Bull. 6 125–130
Hamada T, Antle MC and Silver R 2004 Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur. J. Neurosci. 19 1741–1748
Hardin PE 2011 Molecular Genetic Analysis of circadian timekeeping in Drosophila. The genetics of circadian rhythms. Adv. Genet. 74 141–173
Herzog ED, Hermanstyne T, Smyllie NJ and Hastings MH 2017 Regulating the suprachiasmatic nucleus (SCN) circadian clockwork: interplay between cell-autonomous and circuit-level mechanisms. Cold Spring Harbor Persp. Biol. 9 a027706. https://doi.org/10.1101/cshperspect.a027706
Herzog ED 2007 Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 8 790–802
Hogenboom R, Kalsbeek MJ, Korpel NL, Goede PD, Koenen M, Buijs, R. M, et al. 2019 Loss of arginine vasopressin- and vasoactive intestinal polypeptide-containing neurons and glial cells in the suprachiasmatic nucleus of individuals with type 2 diabetes. Diabetologia 62 2088–2093
Ibata Y, Tanaka M, Ichitani Y, Takahashi Y and Okamura H 1993 Neuronal interaction between VIP and vasopressin neurones in the rat suprachiasmatic nucleus. Neuroreport 4 128–130
Ingram C, Ciobanu R, Coculescu I, Tanasescu R, Coculescu, M and Mihai, R 1998 Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog. Brain Res. Adv. Brain Vasopressin 119 351–364
Ingram C, Snowball R and Mihai R 1996 Circadian rhythm of neuronal activity in suprachiasmatic nucleus slices from the vasopressin-deficient Brattleboro rat. Neuroscience 75 635–641
Irwin RP and Allen CN 2010 Neuropeptide-mediated calcium signaling in the suprachiasmatic nucleus network. Eur. J. Neurosci. 32 1497–1506
Jansen K, Van der Zee EA and Gerkema MP 2000 Being circadian or not: vasopressin release in cultured SCN mirrors behavior in adult voles. Neuroreport 11 3555–3558.
Jansen K, Van der Zee, EA and Gerkema MP 2007 Vasopressin immunoreactivity, but not vasoactive intestinal polypeptide, correlates with expression of circadian rhythmicity in the suprachiasmatic nucleus of voles. Neuropeptides 41 207–216
Jin X, Shearman LP, Weaver DR, Zylka MJ, Vries GJD and Reppert SM 1999 A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96 57–68
Kalsbeek A, Fliers E, Hofman MA, Swaab DF and Buijs RM 2010 Vasopressin and the output of the hypothalamic biological clock. J. Neuroendocrinol. 22 362–372
Kondratova AA and Kondratov RV 2012 The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 13 325–335
Kori H, Yamaguchi Y and Okamura H 2017 Accelerating recovery from jet lag: prediction from a multi-oscillator model and its experimental confirmation in model animals. Sci. Rep. 7, 46702
Kornhauser JM, Mayo KE and Takahashi JS 1996 Light, immediate-early genes, and circadian rhythms. Behav. Genet. 26 221–240
Kraves S and Weitz CJ 2006 A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat. Neurosci. 9 212–219
Kyriacou CP and Hastings MH 2010 Circadian clocks: genes, sleep, and cognition. Trends.Cogn. Sci 14 259–267
Lavebratt C Sjöholm LK Partonen T Schalling M and Forsell Y 2009 PER2 variation is associated with depression vulnerability. Am. J. Med. Genet. B Neuropsychiatric Genet. 153B 570–581
Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo, M et al. 2015 Neuromedin S-Producing Neurons Act as Essential Pacemakers in the Suprachiasmatic Nucleus to Couple Clock Neurons and Dictate Circadian Rhythms. Neuron 85 1086–1102
Lee JE, Zamdborg L, Southey BR, Atkins N, Mitchell JW, Li, M, et al. 2013 Quantitative peptidomics for discovery of circadian-related peptides from the rat suprachiasmatic Nucleus. J. Proteome Res. 12 585–593
Lehman M, Silver R, Gladstone W, Kahn R, Gibson M and Bittman E 1987 Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neurosci. 7 1626–1638
Li J, Burton KJ, Zhang C, Hu S and Zhou Q 2009 Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296 824–830
Li X, Sankrithi N and Davis FC 2002 Transforming growth factor-alpha is expressed in astrocytes of the suprachiasmatic nucleus in hamster: role of glial cells in circadian clocks. Neuroreport 13 2143–2147
Ling HH, Beaulé C, Chiang CK, Tian R, Figeys D and Cheng HY 2014 Time-of-day- and light-dependent expression of ubiquitin protein ligase E3 component N-recognin 4 (UBR4) in the suprachiasmatic nucleus circadian clock. PloS One, 9 e103103
Liou SY and Albers H 1989 Single unit response of suprachiasmatic neurons to arginine vasopressin (AVP) is mediated by a V1-like receptor in the hamster. Brain Res 477 336–343
Loh DH, Kudo T and Colwell CS 2015 Short circuiting the circadian system with a new generation of precision tools. Neuron 85 895–898
Lundkvist GB and Block GD 2005 Role of neuronal membrane events in circadian rhythm generation. Methods Enzymol. Circadian Rhythms 393 623–642
Lundkvist GB, Kwak Y, Davis EK, Tei H and Block GD 2005 A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J. Neurosci. 25 7682–7686
Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan S. K, et al. 2011 Circadian regulation of ATP release in astrocytes. J. Neurosci. 31 8342–8350
Maywood ES, Chesham JE, Obrien JA and Hastings MH 2011 A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc. Natl. Acad. Sci. USA 108 14306–14311
Mcgrath CL, Glatt SJ, Sklar P, Le-Niculescu H, Kuczenski R, Doyle AE, et al. 2009 Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry 9 https://doi.org/10.1186/1471-244x-9-70
Mieda M, Okamoto H, and Sakurai T 2016 Manipulating the cellular circadian period of arginine vasopressin neurons alters the behavioral circadian period. Curr. Biol. 26 18 2535–2542
Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K.-I, Honma S and Sakurai, T 2015 Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 85 1103–1116
Mieda M 2019 The network mechanism of the central circadian pacemaker of the SCN: Do AVP neurons play a more critical role than expected? Front. Neurosci. 13 139. https://doi.org/10.3389/fnins.2019.00139
Mihai R, Coculescu M, Wakerley J and Ingram C 1994a The effects of [arg8] vasopressin and [ARG8] vasotocin on the firing rate of suprachiasmatic neurons in vitro. Neuroscience 62 783–792
Mihai R, Juss T and Ingram C 1994b Suppression of suprachiasmatic nucleus neurone activity with a vasopressin receptor antagonist: possible role for endogenous vasopressin in circadian activity cycles in vitro. Neurosci. Lett. 179 95–99
Mohawk JA, Green CB and Takahashi JS 2012 Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35 445–462
Mohawk JA and Takahashi JS 2011 Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 34 349–358
Moore RY and Eichler VB 1972 Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42 201–206
Moore RY, Speh JC and Leak RK 2002 Suprachiasmatic nucleus organization. Cell Tissue Res. 309 89–98
Moore RY and Speh JC 1993 GABA is the principal neurotransmitter of the circadian system. Neurosci. Lett. 150 112–116
Morel A, Ocarroll AM, Brownstein MJ and Lolaft SJ 1992 Molecular cloning and expression of a rat Via arginine vasopressin receptor. Nature 356 523–526
Morin LP and Allen CN 2006 The circadian visual system, 2005. Brain Res. Rev. 51 1–60
Murphy HM, Wideman CH and Nadzam GR 1998 The role of vasopressin in modulating circadian rhythm responses to phase shifts. Peptides 19 1191–1208
Ng FS, Tangredi MM and Jackson FR 2011 Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr. Biol. 21 625–634
Nielsen HS, Hannibal J and Fahrenkrug J 2002 Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur. J. Neurosci. 15 570–574
Patton AP and Hastings MH 2018 The suprachiasmatic nucleus. Curr. Biol. 28 R816–R822.
Prolo LM, Takahashi JS and Herzog ED 2005 Circadian rhythm generation and entrainment in astrocytes. J. Neurosci. 25 404–408
Reghunandanan V, Badgaiyan R, Marya R and Maini, BK 1987 Suprachiasmatic injection of a vasopressin antagonist modifies the circadian rhythm of food intake. Behav. Neural Biol. 48 344–351
Reghunandanan V, Reghunandanan R, Marya RK and Singh PI 1992 Vasopressin Antagonist Disrupts the Circadian Rhythm of Water Intake on Suprachiasmatic Injection. Chronobiol. Int. 9 356–361
Raghunandanan V, Raghunandanan R and Marya RK 1991 Vasopressin: its possible role in circadian timekeeping. Chronobiologia 18 39–47.
Reghunandanan V, Reghunandanan R 2006 Neurotransmitters of the suprachiasmatic nuclei. J. Circadian Rhythms 4, 2. 10.1186/1740-3391-4-2
Ramkisoensing A and Meijer JH 2015 Synchronization of biological clock neurons by light and peripheral feedback systems promotes circadian rhythms and health. Front Neurol. 6 128
Reppert S, Artman H, Swaminathan S and Fisher D 1981 Vasopressin exhibits a rhythmic daily pattern in cerebrospinal fluid but not in blood. Science 213 1256–1257
Reppert SM and Weaver DR 2002 Coordination of circadian timing in mammals. Nature 418 935–941
Romijn HJ, Sluiter AA, Pool CW, Wortel J and Buijs RM 1997 Evidence from confocal fluorescence microscopy for a dense, reciprocal innervation between AVP-, somatostatin-, VIP/PHI-, GRP- and VIP/PHI/GRP-immunoreactive neurons in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 9 2613–2623
Soria V, Martínez-Amorós È, Escaramís G, Valero J, Pérez-Egea R, García, C, et al. 2010 Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacol 35 1279–1289
Stephan FK and Zucker I 1972 Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 69 1583–1586
Swaab DF, Pool CW, and Nijveldt F 1975 Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophyseal system. J. Neural Transm. 36 195–215
Takahashi JS, Hong HK, Ko CH and Mcdearmon EL 2008 The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9 764–775
Takahashi JS 2016 Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18 164–179
Tso CF, Simon T, Greenlaw AC, Puri T, Mieda, M and Herzog ED 2017 Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr. Biol. 27 1055–1061
Tsuji T, Allchorne AJ, Zhang M, Tsuji C, Tobin VA, Pineda R et al. 2017 Vasopressin casts light on the suprachiasmatic nucleus. J. Physiol. 595 3497–3514
Utge SJ, Soronen P, Loukola A, Kronholm E, Ollila HM, Pirkola S et al.2010 Systematic analysis of circadian genes in a population-based sample reveals association of TIMELESS with depression and sleep disturbance. PloS one 5 e9259
Vadnie CA and McClung CA 2017 Circadian rhythm disturbances in mood disorders: insights into the role of the suprachiasmatic nucleus. Neural plasticity 2017, 1504507. https://doi.org/10.1155/2017/1504507
Van den Pol AN and Tsujimoto K 1985 Neurotransmitters of the hypothalamic suprachiasmatic nucleus: Immunocytochemical analysis of 25 neuronal antigens. Neuroscience 15 1049–1086
Vandesande F, Dierickx K and Mey JD 1975 Identification of the vasopressin-neurophysin producing neurons of the rat suprachiasmatic nuclei. Cell Tissue Res. 156 377–380
Varadarajan S, Tajiri M, Jain R, Holt R, Ahmed Q, Lesauter J and Silver R 2018 Connectome of the suprachiasmatic nucleus: new evidence of the core-shell relationship. Eneuro 5 https://doi.org/10.1523/eneuro.0205-18.2018
Vosko AM, Schroeder A, Loh DH and Colwell CS 2007 Vasoactive intestinal peptide and the mammalian circadian system. Gen. Comp. Endocrinol. 152 165–175
Wang MH, Chen N and Wang JH 2014 The coupling features of electrical synapses modulate neuronal synchrony in hypothalamic suprachiasmatic nucleus. Brain Res. 1550 9–17
Welsh DK, Takahashi JS and Kay SA 2010 Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 72 551–577
Wideman CH, Murphy HM and Nadzam GR 2000 Vasopressin deficiency provides evidence for separate circadian oscillators of activity and temperature. Peptides 21 811–816
Womac AD, Burkeen JF, Neuendorff N, Earnest DJ and Zoran MJ 2009 Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur. J. Neurosci. 30 869–876
Wu X, Balesar R, Lu J, Farajnia S, Zhu Q, Huang M et al. 2017 Increased glutamic acid decarboxylase expression in the hypothalamic suprachiasmatic nucleus in depression. Brain Struct. Funct. 222 4079–4088
Yamaguchi S, Isejima H, Matsuo T, Okura R, Kobayashi M, Okamura H et al. 2003 Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302 1408–1412
Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y et al. 2013 Mice genetically deficient V1a and V1b receptors are resistant to jet lag. Science 342 85–91
Yan L, Takekida S, Shigeyoshi Y and Okamura H 1999 PER1 and PER2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience 94 141–150
Yoshikawa T, Nakajima Y, Yamada, Y, Enoki R, Watanabe K, Yamazaki M et al. 2015 Spatiotemporal profiles of arginine vasopressin transcription in cultured suprachiasmatic nucleus. Eur. J. Neurosci. 42 2678–2689
Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJG, Heerikhuize JJV, Hofman MA et al. 2001 Alterations in Arginine Vasopressin neurons in the suprachiasmatic nucleus in depression. Arch. Gen. Psych. 58 655
Acknowledgements
The author would like to thank Mrs Reshmi Menon Sharma for her valuable help and support at various stages of the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Aurnab Ghose
Rights and permissions
About this article
Cite this article
Reghunandanan, V. Vasopressin in circadian function of SCN. J Biosci 45, 140 (2020). https://doi.org/10.1007/s12038-020-00109-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-020-00109-3