Abstract
The paper discusses the applicability and advantages of using electrically charged soap bubbles as a route to produce fine sprays composed of highly charged particles, commonly named as electroaerosols. It is indicated that such low in energy demand process may produce very fine droplets or particulates charged to a level higher than that obtained using classical spray charging techniques, involving no bubbles. A process of a soap bubble electrical charging is thus initially studied on a simple analytical basis pointing out to a possibility of producing air bubbles with charge-to-mass (Q/m) ratio up to 60 mC/kg (constrained by the Rayleigh limit) while just 2 mC/kg is commonly considered as a threshold value for an effective particle charging process. Finite element 3D electrostatic simulation method (3D FEM) is then applied to assess a single bubble charging level achievable in a laboratory setup using a DC high-voltage biased bubble machine producing soap bubbles 23 mm in diameter on average. The 3D FEM simulation results postulate a single bubble maximum charging level approaching 25 nC at 41.5 kV charging voltage (constrained by the Rayleigh limit), corresponding to (Q/m) = 17.6 mC/kg. Finally, a stream of bubbles characterized by (Q/m) = 10.4 mC/kg was produced experimentally in the laboratory setup using a contact charging method at 40 kV DC supply. A discrepancy between 3D FEM-simulated results and experimental data was discussed on a shielding effect basis.
Graphic abstract

Similar content being viewed by others
1 Introduction
Electroaerosols specified as sprays composed of charged particles have been recently extensively studied as well as broadly utilized in many industrial processes including crop spraying, pollination, spray painting, electrospray air cleaning, and pharmaceutical inhalation, just to name a few. The main advantage of having either liquid droplets or powdered solids charged involves the possibility of controlling their trajectory along the electric field lines (Chang et al. 1995; Khan et al. 2012; Jaworek et al. 2018). Although every charged particle can be in theory controlled this way, yet only those charged over some limit in relation to their mass are of practical importance. Thus, charge-to-mass ratio (Q/m) is the commonly concerned quantity describing aerosol charging effectiveness and (Q/m) = 2 mC/kg is considered as a threshold value for an effective particle charging process (Chang et al. 1995).
Although various spray charging techniques have been developed, relying in general either on induction charging of particles directly during their atomization (applicable for conducting or semi-conducting sprayed materials) or post-atomization corona charging using ions, there is a constant need to search for methods to improve the electroaerosol generation effectiveness, either in terms of the mass rate production or increased (Q/m) value. Producing charged aerosol from bursting bubbles seems to be a very advantageous approach if those two factors are concerned. The bubble-engaged electroaerosol production method was proposed by Minardi et al. (1981) for an electrohydrodynamic ion wind generator and its idea may be outlined as follows. A stream of constantly generated (using any available method) soap bubbles is electrically charged either during- or their post-formation. Separated charged bubbles are then transported away and undergo either spontaneous or forced bursting. After bursting, bubbles emit descendant droplets having broad diameter distribution ranging from tenths of nm to hundreds of μm (Ke et al. 2017; Lhuissier et al. 2012; Modini et al. 2013) making a charged liquid aerosol.
The main advantage of the charged aerosol formation involving the bubble step is related to much higher possible (Q/m) ratios than achieved in case of a direct droplet formation and charging. In the case of a charged droplet, because of the Coulomb force, the free net charge is located principally on its surface (Doyle et al. 1964). Thus, only its surface provides the charge factor but the whole droplet volume contributes to the mass factor. On the other hand, a bubble can acquire the same charge but as it is composed only of a thin liquid shell (of the order of 0.1–1 μm), it is considerably lighter than the droplet with the same radius. Therefore, much higher (Q/m) values can be achieved in the case of charged bubbles. In general, bubble production requires also much lower fluid flow rates than droplet production (especially in case of large bubbles) which significantly reduces energy demands in case of the bubble-electroaerosol making process (Omer et al. 2011; Salkin et al. 2016; Davison et al. 2017). The bursting of bubbles occurs naturally due to film drainage (Ke et al. 2017), so in principle, it does not require externally supplied energy. Moreover, it is expected that highly charged bubbles should burst spontaneously (due to field-induced bubble instabilities and Rayleigh limit surpassing leading to local partial discharges emanating from the bubble film). Furthermore, when ruptured they ought to produce finer secondary droplets (than in case of uncharged bubbles) due to strong and repulsive Coulomb force interactions in the fragmenting liquid film and between the resulting homo-charged liquid drops. Besides, if a suitable solution comprised of dissolved solid(s) is used to make highly charged bubbles, it becomes possible to produce highly charged and very fine particulates due to liquid spray drying.
Charged bubbles have already been applied in modeling of ball lighting dynamics (Stephan 2008) and, in recent years, interest in bubble-electrospinning technology has also become apparent (Liu et al. 2008; Ren et al. 2011). A single soap bubble charging process was also investigated in our previous study (Pelesz 2018). It was found that a high-voltage direct contact charging of a single bubble allows reaching the charge limit resulting in either its instantaneous bursting or self-splitting into two daughter-bubbles. The average (Q/m) parameter reaching 3.4 mC/kg was found to be representative for such bubbles; however, it was limited mainly by a relatively large mass of the implemented bubbles.
Considering the aspects mentioned above, producing charged aerosol from bursting bubbles may be an effective and energy-efficient alternative method. On the other hand, it poses also certain disadvantages, including the unpredictability of bubble rupture moment as well as the wide size distribution of the aerosol droplets. For the above reasons, there is a need to experimentally study the basic bubble charging process as well as a behavior of already-charged bubbles.
2 Analytical motivation for the experiment
The following simple and straightforward analytical outline lays out the background for the subsequent experimental research as well as it lets us calculate quantities meaningful for the FEM simulation and laboratory tests and relate each other.
Table 1 lists all symbols used in the following calculations.
If we assume a thin-walled bubble, its mass can be calculated using a straightforward formula:
However, in reality, a bubble film thickness d is difficult to be established as it is dependent—among other factors—on the bubble radius rb and such exact analytical dependence is undetermined. On the other hand, if a dependency between a bubble radius and its mass is known it can be combined with (1) and thus an estimated relationship between a bubble radius and its film thickness can be articulated. Therefore, using literature-provided experimentally examined mass data (Torikai et al. 2011) for soap bubbles ranging from 25 to 55 mm in radius (indicated in Fig. 1 by black-filled circles) the regression-approximated mass–radius bubble dependency can be expressed as an empirical relation:
where mb is given in (kg) and rb is in (m) and the power-type regression model used in (2) provides the best fit in terms of R2 (the goodness of fit) approaching 0.9951 (as revealed in Fig. 1). If we suppose that the physic of bubble formation is not seriously flawed in case of bubbles smaller than 50 mm in diameter, Eq. (2) can be used to extrapolate bubble mass–radius data down to 5 mm (as indicated in Fig. 1 by empty circles). Finally, combining (1) and (2) and rearranging it provides the following relation:
where d and rb is given in (m) and ρ in (kg/m3).
Evaluating (3) numerically for a commonplace density of a bubble solution (ρ = 998 kg/m3, a value representative for the liquid used in the experimental part of the study) it can be finally concluded that a bubble film thickness observed experimentally is expected to be ranging from approx. 490 nm for small bubbles (10 mm in diameter) up to 755 nm for larger bubbles (110 mm in diameter). Such estimated film thickness agrees well with the range of typical bubble film thickness as discussed and examined experimentally by Ke et al. (2017). Thus, it is reasonable to consider (2) and (3) as adequately accurate in the context of the experimental work carried out. Moreover, it should be indicated that the bubble mass–radius dependency may be also related to the bubble making process and the involved equipment (which cannot be straightforwardly accommodated into an equation) as well as to properties of the employed soap solution and the atmosphere filling and surrounding a bubble (i.e., the surface tension). However, such deep theoretical study is not within the scope of the presented experimental research aiming at the soap bubble charging.
Therefore, if the electrical charging of bubbles is considered it should be pointed out that the main factor limiting the bubble maximum charging level (especially important from a practical point of view) is related to the equilibrium between the electrostatic and excess pressure determined by the surface tension γ of the bubble liquid. If the electrostatic pressure (acting outwards) is higher than the excess pressure, the surface of the bubble will become unstable and it will rupture thus restricting further charging level increase. This progression is analogous to a single liquid droplet charging process in which the pressure imbalance initiates its subsequent divisions into secondary drips until the pressure balance is restored. Hence, for an idealized spherical droplet with radius rd, the balance between those two pressures can be written as
in which the left side represents the electrostatic pressure whereas the right side denotes the excess pressure. As the total charge accumulated on a spherical object can be calculated using a well-known electric filed theorem:
thus, the maximum electrical charge attained by a spherical droplet can be determined by combing (4) and (5) into
which is a foundation of the estimate well known as the Rayleigh limit (Rayleigh 1882).
For a spherical bubble, which effectively has two surfaces, the balance between electrostatic and excess pressure can be written as (Isenberg 1978)
and thus the Rayleigh limit for a spherical bubble yields
By comparing (6) and (8), it becomes evident that a bubble can collect approx. 41% more charge than an analogous droplet with the same radius. Thus, charging of bubbles should presumably yield higher charging level than attainable for droplets of the same liquid.
Figure 2 illustrates the maximum bubble charge calculated numerically using (8) for the literature-selected (Ke et al. 2017; Cohen et al. 2017) extreme (20 and 50 mN/m) and average (35 mN/m) surface tension γ values representative for commonplace soap bubble solutions. It becomes evident that the maximum bubble charge may thus rise over 300 nC if large bubbles (exceeding 110 mm in diameter) are formed using a liquid with high surface tension (50 mN/m). On the other hand, in the case of bubbles 20 mm in diameter, the maximum charge can come about 15–25 nC in the whole asserted 20–50 mN/m surface tension range.
Maximum bubble charge Qb.max, calculated using (8) for a range of the surface tension γ values typical for commonplace bubble solutions
Another principal factor limiting the maximum charge acquired by a bubble is the dielectric strength of the surrounding medium. In case of air, it is assumed that if the electric field strength on the surface of a spherical object exceeds 3 MV/m it will cause a partial discharge in the object gaseous vicinity resulting in a definite charge loss (Chang et al. 1995). The maximum strength of the electric field creäted by a single electrically charged bubble can be thus expressed by rearranging (5) into
The maximum electric field strength on the surface of a single bubble Emax can be consequently calculated by inserting Eq. (8) directly into (9) and as such it is numerically presented in Fig. 3 as a bubble radius dependency. The numerical calculations of Emax were again parametrized by the extreme and average surface tension γ values of a bubble solution (20, 50 and 35 mN/m respectively, as in case of Qb.max computation). Thus, it may be expected that Emax exceeds 3 MV/m only in case of bubbles with a radius smaller than 5 mm and only for a soap solution of the highest (50 mN/m) surface tension. Such observation advocates the data extrapolation ranging down to 5 mm, as discussed before and shown in Fig. 1. As a consequence, the dielectric strength of the air becomes a limiting factor only in case of very small bubbles made of a particularly high surface tension liquid. Moreover, the maximum charging level in small bubbles may be further increased if the atmosphere with higher dielectric strength is used.
Finally, the maximum feasible value of the charge-to-mass ratio (Q/m)max characteristic for a bubble may be calculated directly by combining (1) and (8) as follows:
Although Eq. (10) allows calculating (Q/m)max value, it still requires knowledge of the bubble film thickness d. On the other hand, such difficulty may be eliminated if (Q/m)max is calculated ex definitione, i.e., if Qb max given by (8) is divided by a regression-estimated mass factor given by (2). Thus, without sacrificing an experimentally required accuracy, we finally obtain a numerical estimate of (Q/m)max in the following form:
where rb is given in (m), γ in (N/m) and ε0 in (F/m).
Figure 4 illustrates values of (Q/m)max calculated using (11) and parametrized by the extreme and average surface tension γ values (20, 50 and 35 mN/m, respectively) in the same way as in case of Qb.max calculation.
Maximum (Q/m) ratio [calculated using (11)] for a single bubble with radius rb
Analyzing (11), it may be concluded that the maximum (Q/m) ratio is inversely proportional to the bubble radius thus, smaller bubbles may reach substantially higher (Q/m) ratio, especially those made of a liquid with high surface tension. It should be also pointed out that even relatively large bubbles (rb = 50–55 mm) may obtain applicably high (Q/m) values exceeding 7 mC/kg, irrelevantly on the surface tension (ranging from 20 to 50 mN/m). Furthermore, obtaining (Q/m) values higher than 10 mC/kg should be possible if bubbles with a radius smaller than 30 mm are handled.
All the above-discussed calculations should be considered as a coarse estimate. However, even if we assume that real bubbles are twice heavier than it appears from (2) and thus their maximum (Q/m) ratio is 2 times smaller than the value envisaged by (11) it will be still high enough to justify carrying out experimental research related to the charged bubble spraying method.
3 Measurement setup
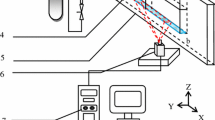
All laboratory measurements were performed in the setup schematically presented in Fig. 5. It consisted of a commercial bubble machine (BM) with its liquid container connected directly to a DC high voltage source (PS/EW50P12, Glassman). The collecting electrode CE was made of a thin aluminum foil laid inside a lightweight box made of a thin-walled foamed polystyrene sheet. The total mass of the insulating box and the electrode yielded 57.7 g. The box with the electrode was placed on an electronic weighing scale and located in such position as to collect bubbles. Collecting electrode was connected to a pico-ammeter (Keithley 485) using a flexible metallic strip and a shielded cable. Thus, charging method used in the experiment can be described as two-electrode conduction method, in which the liquid at high potential was acting as one electrode and the collecting electrode (effectively grounded by the pico-ammeter shunt resistance) was the second one (Pelesz et al. 2020).
Schematic representation of the measurement setup (HVDC high-voltage DC supply, BM bubble machine, L liquid container, HSC high-speed camera, BSt bubble stream, CE collecting electrode (grounded), INS foamed polystyrene insulator, W electronic weighing scale, pA pico-ammeter, OSC oscilloscope, PC computer)
The voltage output of the pico-ammeter was connected to a digital oscilloscope (Tektronix DSO 2014) and the scope, as well as the weighing scale, was interfaced to a personal computer for automated data acquisition. A high-speed digital camera (fps1000, SMCCL) was positioned near the bubble machine nozzle to visually observe and record the bubble formation process.
The bubbles were produced using a commercial soap solution (density ρ = 998 kg/m3, electrical conductivity σ = 7 mS/cm, and surface tension γ = 34 mN/m as determined by the capillary rise method). All experiments and measurements were carried out in regular indoor air conditions (temperature 22 ± 3 °C, relative humidity 40 ± 5% RH).
Visual inspection of the picture showing a sequence of soap bubbles emerging from the bubble machine nozzle (presented in Fig. 6) ascertains that the bubble making process employed in the discussed experiments does not differ from that described in the literature (Salkin et al. 2016, Davidson et al. 2017). The bubble machine employed in the experiments was capable of producing 116 bubbles per second on average; the bubbles were matched in size and had a mean radius of 11.7 ± 1.8 mm. As 10 g of the soap solution was consumed per minute so the average mass of a single bubble amounted to 1.4 mg, i.e., almost twice as much as expected using (2). Thus, the average thickness of the bubble shell, calculated using (1), would amount to approx. 800 nm and the expected maximum bubble charge, calculated using (8) would yield 22.5 nC corresponding to the maximum (Q/m) = 16.1 mC/kg. Such high (Q/m) value hypothetically attainable in the above-described laboratory experiment is over 8 times higher than a threshold value (2 mC/kg) for an effective particle charging process, which makes the bubble charging route very attractive in the electroaerosol making context.
4 FEM simulations
Purely electrostatic finite element method (FEM) simulation is sufficient to correctly predict the charge magnitude in case of a droplet induction or conductive charging performed in a real experimental arrangement (Pelesz et al. 2020) as well as it also provides the numerical link between the bubble charge and the magnitude of the voltage correlated to the electric field. Thus, to computationally estimate the voltage dependence and the maximum value of the charge attained by bubbles electrified in the above-discussed experimental setup, a simplified geometrical model representing the real laboratory system geometry (schematically shown in Fig. 5) was designed. The 3D model was composed of a bubble machine, a soap bubble in its final stage of growth (i.e., just before its separation from the bubble machine nozzle) and a collector electrode located in a dielectric box, as schematically presented in Fig. 7. Those three objects were located in the center of a cylindrical space (2.4 m in diameter and 2.0 m high) synthetically reproducing the air-filled (the relative dielectric constant εr = 1) close surrounding of the bubble machine and the collecting electrode. The boundary condition set at the external walls of this cylindrical space assumed only the tangential component of the electric field (thus reproducing all electrically grounded neighboring entities).
BM simulation model is closely represented in Fig. 8a and its dimensions closely reflected the size of the real BM employed in the experiment (height 135 mm, length 100 mm, and width 45 mm). The plastic BM casing was modeled as a solid dielectric body (with the relative dielectric constant εr = 2) whereas a semi-conductive bubble solution contained in BM and acting as the HV electrode was modeled as detailed in Fig. 8b. Bubble solution was embodied in the 3D model as the volume present in the liquid container (the lower cylindrical part of BM), the internal liquid feed line as well as the just-detaching bubble shell, modeled as detailed in Fig. 8c. The bubble shell model was balloon-shaped to approximate the real profile of the bubble forming at the BM nozzle exit, as evidenced in Fig. 6. The fold-up formation, find out in Fig. 6, which directly preceded a bubble detachment was not hosted in the FEM model. Instead, it was assumed that the total charge acquired by the detaching bubble was equal to the charge induced on its whole frontal and fraction of its rear part as marked by a hashed region in Fig. 8c. The surface of the marked region was equivalent to the surface of the fully developed spherical bubble detached from the BM nozzle. Such an approach reflected the charge inflow right at the moment of the bubble detachment.
The charge Qi induced on the above-stated bubble shell fragment and thus finally acquired by a completely formed and detached bubble was FEM-simulated as a function of the charging voltage Ue and its progression is represented in Fig. 9. Because the induced charge is directly proportional to the electric field strength in the vicinity of the bubble shell, and because the simulated electric field distribution is independent on the charging voltage level thus consequently the dependence of the induced charge Qi on Ue is linear and the FEM-calculated slope of this dependency equals to 0.595 nC/kV. However, in real experimental conditions, the formation of bubbles and the shape of a developing bubble are field dependent and thus the real Qi value may depend nonlinearly on Ue.
The induced charge Qi obtained in the FEM simulation is thus equal to the Rayleigh limit Qb max = 24,67 nC at the charging voltage Ue = 41.46 kV (the Rayleigh limit is predicted at this point using (8) for the authentic γ = 34 mN/m and rb = 11.7 mm values noted for real experimentally generated bubbles and its value is indicated in Fig. 9 by a horizontal dashed line). If the experimentally determined single bubble averaged mass (1.4 mg) is also taken into account the induced charge Qi = 24.67 nC corresponds to (Q/m)max = 17.6 mC/kg. Those FEM-predicted Qi and (Q/m)max values are thus comparable to the same quantities elaborated based on the previously analytical-derived equations, as discussed in the last paragraph of the Measurement setup chapter. Moreover, if such utmost FEM-anticipated charging level was indeed achieved in a real laboratory experiment, it would be expected to observe an expansion of the bubble size due to Coulomb force-related overpressure or even a spontaneous bursting of the bubbles (due to a statistical spread of rb values observed in the conducted experiment).
5 Experimental results
An experimental record of the bubble collected mass for the uncharged stream of bubbles is shown in Fig. 10. As soon as the bubbles started reaching the CE electrode, the collected mass began increasing linearly at the rate of 74.5 mg/s as the bubble machine yield was fairly constant over time. The observed collection rate was smaller than the bubble machine mass yield (1.67 g/s) as only approx. 45% of the produced bubbles were collected by the collecting electrode (while the rest departed and deposited in the surrounding area). After pausing the bubble machine operation, the collected mass remained constant for about 8 s. Afterwards, a sudden decrease in the collected mass was observed, related to natural bursting of bubbles resulting in the ejection of daughter droplets outside the collecting box. After that, the accumulated fluid mass slowly decreased linearly over time due to water evaporation at a mean rate of 1.9 mg/s.
Exemplary record of the bubble mass accumulation for the charged bubble stream (at the highest experimentally possible charging voltage Ue = 40 kV) is presented in Fig. 11. In this case, bubbles were collected at a mean mass rate of 57.4 mg/s which was smaller than that observed for the uncharged bubble stream. As the charging voltage increases, more and more bubbles are escaping away from the collecting electrode due to the repelling effect of the space charge cloud formed by the bubble diverging stream hovering over the CE box. Therefore, the collection effectiveness at 40 kV dropped to approx. 34%. On the other hand, in the real experimental conditions, the charging voltage was limited to 40 kV because serious corona discharges were noticed in the bubble machine section inhibiting further voltage escalation.
It should be noted that an apparent decrease in the accumulated bubble mass is noticed in the initial stage of the experiment involving the charged bubbles (as in the left part of Fig. 11). This transient declining mass fluctuation was not an experimental fault. Instead, it was brought about by the electrostatic attraction between the charged bubble cloud floating in the air and the corresponding image charge induced at the grounded collecting electrode located on the weighing scale. The attractive force Fe exerted on the CE electrode due to the bubble cloud space charge may be assessed simply using the 2nd dynamics principle as follows:
where mΔ is an apparent mass decrease and the positive sign is used in (12) as the electrostatic force is acting upwards against the gravity. Fe calculated using (12) at each of the experimentally applied charging voltage level is plotted in Fig. 12. It should be pointed out that Fe is proportional to the charging voltage Ue2 squared as it is related to the Maxwell attracting electrostatic stress appearing between the bubble cloud and its image charge and acting on the collecting electrode effectively pulling it upwards. However, this effect only biased the mass readings by a constant amount and as the bubble mass accumulation rate was used in all subsequent calculations such bias was inevitably neglected.
Finally, the bubble mass build-up rate ke recorded at the collecting electrode was determined as a function of the charging voltage Ue and it is illustrated in Fig. 13. The highest bubble mass build-up rate was observed for the uncharged bubble stream but as the charging voltage was increasing the mass build-up rate initially decreased but at a certain charging voltage it started to increase again. To explain this dual trend it should be noted that there are two sources of electrostatic forces acting on each bubble. The first one is related to the attraction of bubbles to the grounded CE electrode due to the image charge effect (discussed above), whereas the second one is associated with the mutual repulsion of mono-polar charged bubbles within the bubble cloud (in particular those floating directly over the collector electrode). Combination of these two effects was responsible for characteristic curve shape seen in Fig. 13. On average, the repulsive force, steering some number of the bubbles away from the collecting electrode (and thus reducing its collecting effectiveness) started to be counterbalanced by the attracting force exceeding approx. 0.2 mN (as observed in Fig. 12 at Ue = 15 kV), related to the image charge induced in CE at the charging voltage surpassing 15 kV (i.e., at the minimum of the data in Fig. 13).
As the experimentally observed charge build-up was semi-linear over time the corresponding collector electrode mean current value was constant for a given charging voltage. The experimentally observed (nor shown here for clarity) local deviations from a straight Q–t line were related to the discrete and chaotic character of the charge transport involving charged bubbles. The bubbles were reaching the collecting electrode at randomly scattered time intervals due to minuscule instabilities in the bubble creation process (proceeding at the bubble machine nozzle) and complex interactions between bubbles in the bubble stream. The mean current im related to the captured charged bubble stream was nonlinearly increasing with Ue with minor local sways at approx. 15 kV and 30 kV, which were in coincidence with aberrations in the bubble mass build-up rate, discussed before.
Finally, the (Q/m) parameter, calculated using experimentally established mass accumulation rate and the mean current values is illustrated in Fig. 14. The experimentally evaluated relation (Q/m) = f(Ue) was quasi-linear with a slope equal to 0.242 mC/kg/kV whereas the maximum (Q/m)max value of 10.4 mC/kg was reached at Ue = 40 kV.
Still photographs of the bubble stream taken at three diverse charging voltage levels (no voltage, 20 kV, and 40 kV) are presented in Fig. 15. The uncharged bubbles formed a compact, slightly diverging jet, in which certain (heavier) bubbles tend to fall due to gravitational force overcoming airstream drag. Such process escalated as the bubbles moved away from the nozzle into the region with reduced airstream velocity (Fig. 15a). In the case of the charged stream, it was evident that the bubbles repel each other due to the action of repulsive electrostatic forces present in homo-charged bubble cloud (Fig. 15b, c). However, even for the highest charging voltage applied (Fig. 15c), no spontaneous bubble bursting was detected. The radius of bubbles was slightly and non-monotonically dependent on the charging voltage, only the spread of radius values tend to increase with the charging voltage (11.7 ± 1.8 mm at Ue = 0; 12.9 ± 2.7 mm at 20 kV, and 12.2 ± 4.5 mm at 40 kV on average). The above remarks indicate that the charge induced on the bubbles in the real experiment was not approaching the limit analytically envisaged by (8) or estimated using FEM electric field stimulation (as shown in Fig. 9).
Knowing the average mass of a bubble (1.4 mg) the mean charge of a single bubble was finally estimated as a function of Ue using experimentally determined (Q/m) ratio and it is marked in Fig. 16 using filled circles. Figure 16 is also supplemented with the mean bubble charge calculated numerically in FEM simulation (as already shown in Fig. 9) for easy comparison.
The experimentally determined single bubble charge characteristic Qb = f(Ue) was semi-linear with a slope of 0.339 nC/kV while the FEM-determined dependency had a slope of 0.595 nC/kV. The experimentally determined value of the single bubble charge was thus on average 43% smaller than the numerically FEM-simulated value. Such discrepancy between the experimental results and FEM-simulated values may be most likely related to the shielding effect (Kacprzyk et al. 2011; Osman et al. 2015). The real bubble stream leaving the bubble machine nozzle was very compact and the distance between the first already detached bubble and the one being just formed (and thus acquiring the charge in the induction process) was relatively small (3 mm on average) in comparison to the bubble radius (exceeding 12 mm on average as seen in Fig. 15b, c at 20 and 40 kV). Moreover, due to a low airstream velocity exploited by the bubble machine, the bubbles which were already formed and departed produced a cone-shaped space charge cloud present in the front of the bubble machine nozzle (as seen in Fig. 15). The performed FEM simulation did not account for those two phenomena causing electrostatic shielding of the rising bubble, weakening the near-bubble field strength and thus reducing the charge induced on the bubble. As a consequence, the real electric field strength present nearby the bubble expanding at the nozzle was lower than the FEM-simulated estimate.
Moreover, the maximum charge (14.6 nC) acquired by the bubbles in the experiment has reached approx. 56% of the Rayleigh limit (26.3 nC) envisaged by (8) for the bubble mean radius 12.2 mm (noted at 40 kV) and the soap solution surface tension 34 mN/m. This fact explains why no bubble bursting or an expressive difference in the behavior of bubbles with increasing charging levels was observed (apart from stronger repulsion noted in the bubble stream at higher charging levels as illustrated in Fig. 15b, c).
6 Conclusions
The vital parameters characteristic for the charged bubbles discussed in the current paper in comparison to values determined previously in soap bubbles contact charging experiments, as debated in detail in our former paper (Pelesz 2018), are brought together in Table 2.
It should be noted that in the previous study, bubbles were produced manually and thus their size and mass reproducibility was quite humble (Pelesz 2018). The hand-produced bubbles ranged in radius from 15 to 25 mm which corresponded to Qb∙max in the range 36–78 nC [as calculated using (8)]. Thus, the mean experimentally observed bubble charge of 45 nC was within the analytically envisaged range. The Rayleigh charge limit, resulting in the bubble self-bursting was reached because the shielding effect was not present in the previous experiments. As a single bubble was slowly formed at each time and it was charged individually by a direct contact method, the shielding effect could not have occurred. On the other hand, a mass of those man-made bubbles was fluctuating from 9 to 24 mg, whereas the corresponding size-dependent bubble mass calculated using (2) should have encompassed just 1.7–5.4 mg. Consequently, the previously hand-made bubbles were 4–5 times heavier than their analytical estimates, resulting in relatively low mean (Q/m) value of 3.4 mC/kg (whereas the individual (Q/m) values were scattered from approx. 2 to 5 mC/kg). Contrary, the sequentially machine-produced soap bubbles, implemented in the presently documented experiments were closely matched and reproducible in size and mass. Even though a dynamic charging process accompanying an abrupt bubble formation was not so effective as the direct and slow contact charging used in the previous study (Pelesz 2018) and resulted in the bubble charge almost 1.7 times smaller than the estimated Qb max charge limit, but the actual (Q/m) parameter was exceeding 10 mC/kg. Thus, it was almost three times higher than the previously noted mean value (Pelesz 2018) and over five times higher than the value (2 mC/kg) considered as a threshold for an effective charging process. The laboratory-determined (Q/m) values exceeding 10 mC/kg for the charged bubbles is a noteworthy experimental achievement, not referenced in the literature so far. Moreover, currently discussed experiments demonstrated a clear difference in the movement trajectory between the electrified bubbles and the neutral ones evidencing that the presently achieved (Q/m) value was high enough to ensure the dominance of the electrostatic forces over gravitational pull-in.
These appealing results indicate that the proposed bubble-route aerosol charging method is promising and needs further research and development. As the obtained bubble charge was about two times smaller than that anticipated by the Rayleigh limit, the shielding effect in the soap bubble stream needs to be closely examined and possibly eliminated or at least reduced. Future works should thus focus on developing a numerical FEM model taking into account the shielding effect as well as on optimizing the overall electrification conditions to maximize (Q/m) values at voltages significantly lower than those implemented in the current paper. Helium-filled soap bubbles (HFSB) technology capable of producing thousands of sub-millimeter bubbles per second (Scarano et al. 2015; Bosbach et al. 2009; Faleiros et al. 2019) could be, therefore, tested for applicability. Moreover, the development of a reliable and effective bubble bursting method delivering reproducible nanometer-in-size aerosol droplets is required if the aerosol making is considered.
References
Bosbach J, Kühn M, Wagner C (2009) Large scale particle image velocimetry with helium-filled soap bubbles. Exp fluids 46(3):539–547
Chang J-S, Kelly AJ, Crowley JM (1995) Handbook of electrostatic processes. CRC Press, Boca Raton
Cohen C et al (2017) On the shape of giant soap bubbles. Proc Nat A Sci 114(10):2515–2519
Davidson J, Sangjin R (2017) High-speed visualization of soap bubble blowing and image-processing-based analysis of pinch-off dynamics. J Vis 20(1):53–61
Doyle A, Moffett DR, Vonnegut B (1964) Behavior of evaporating electrically charged droplets. J Colloid Sci 19(2):136–143
Faleiros DE et al (2019) Generation and control of helium-filled soap bubbles for PIV. Exp. Fluids 60(3):40
Isenberg C (1978) The science of soap films and soap bubbles, ch 1.4. Tieto, Clevedon, pp 13–17
Jaworek A, Sobczyk AT, Krupa A (2018) Electrospray application to powder production and surface coating. J Aerosol Sci 125:57–92
Kacprzyk R, Zylka P (2011) Electrification of aerosol particles in supersonic atomizers. IEEE Trans Dielectr Electr Insul 18(5):1353–1360
Ke W-R et al (2017) Characterization of aerosol emissions from single bubble bursting. J Aerosol Sci 109:1–12
Khan MKI et al (2012) Electrostatic powder coating of foods—state of the art and opportunities. J Food Eng 111(1):1–5
Lhuissier H, Villermaux E (2012) Bursting bubble aerosols. J Fluid Mech 696:5–44
Liu Y, He J-H, Yu J-Y (2008) Bubble-electrospinning: a novel method for making nanofibers. J Phys Conf Ser 96(1):012001
Minardi JE, Lawson OM, Wattendorf FL (1981) Fourth Annual Progress Report on the Electrofluid Dynamic Wind Generator: Final Report for the Period 1 April 1979–31 August 1980, Nat. Renewable Energy Lab. (NREL), Golden, CO, USA, no. SERI/TR-9-8074-1
Modini RL et al (2013) Effect of soluble surfactant on bubble persistence and bubble-produced aerosol particles. J Geophys Res Atmos 118(3):1388–1400
Omer K, Ashgriz N (2011) Spray Nozzles. In: Ashgriz N (ed) Handbook of atomization and sprays. Theory and applications, ch. 24. Springer, New York, pp 497–602
Osman H et al (2015) The charging level of a ligament-droplet system atomized in a uniform electric field. IEEE Trans Ind Appl 52(2):1814–1822
Pelesz A (2018) Charging of a single soap bubble. Przegl Elektrotech 94(10):176–179
Pelesz A, Czapka T (2020) Empirical and numerical analysis of conduction and induction charging of droplets in a three-electrode system. Energies 13(2):469
Rayleigh FRS (1882) XX. On the equilibrium of liquid conducting masses charged with electricity. Philos Mag (1798–1977) 14(87):184–186
Ren Z-F, He J-H (2011) Single polymeric bubble for the preparation of multiple micro/nanofibers. J Appl Polym Sci 119(2):1161–1165
Salkin L et al (2016) Generating soap bubbles by blowing on soap films. Phys Rev Lett 116(7):077801
Scarano F et al (2015) On the use of helium-filled soap bubbles for large-scale tomographic PIV in wind tunnel experiments. Exp Fluids 56:42
Stephan KD (2008) Electrostatic charge bounds for ball lightning models. Phys Scr 77(3):035504
Torikai H et al (2011) Extinguishment of a laminar jet diffusion flame using a soap bubble filled with nitrogen gas. Fire Saf Sci 10:557–567
Funding
This work was supported within the subvention of the Department of Electrical Engineering Fundamentals of the Wroclaw University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pelesz, A., Zylka, P. On analytical and experimental aspects of soap bubble stream charging. Exp Fluids 61, 241 (2020). https://doi.org/10.1007/s00348-020-03078-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00348-020-03078-3