Abstract
Overexpression of EGFR drives glioblastomas (GBM) cell invasion but these tumours remain resistant to EGFR-targeted therapies such as tyrosine kinase inhibitors (TKIs). Endocytosis, an important modulator of EGFR function, is often dysregulated in glioma cells and is associated with therapy resistance. However, the impact of TKIs on EGFR endocytosis has never been examined in GBM cells. In the present study, we showed that gefitinib and other tyrosine kinase inhibitors induced EGFR accumulation in early-endosomes as a result of an increased endocytosis. Moreover, TKIs trigger early-endosome re-localization of another membrane receptor, the fibronectin receptor alpha5beta1 integrin, a promising therapeutic target in GBM that regulates physiological EGFR endocytosis and recycling in cancer cells. Super-resolution dSTORM imaging showed a close-proximity between beta1 integrin and EGFR in intracellular membrane compartments of gefitinib-treated cells, suggesting their potential interaction. Interestingly, integrin depletion delayed gefitinib-mediated EGFR endocytosis. Co-endocytosis of EGFR and alpha5beta1 integrin may alter glioma cell response to gefitinib. Using an in vitro model of glioma cell dissemination from spheroid, we showed that alpha5 integrin-depleted cells were more sensitive to TKIs than alpha5-expressing cells. This work provides evidence for the first time that EGFR TKIs can trigger massive EGFR and alpha5beta1 integrin co-endocytosis, which may modulate glioma cell invasiveness under therapeutic treatment.





Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Brennan CW, Verhaak RGW, McKenna A et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. https://doi.org/10.1016/j.cell.2013.09.034
An Z, Aksoy O, Zheng T et al (2018) Epidermal growth factor receptor (EGFR) and EGFRvIII in glioblastoma (GBM): signaling pathways and targeted therapies. Oncogene 37:1561–1575. https://doi.org/10.1038/s41388-017-0045-7
Taylor TE, Furnari FB, Cavenee WK (2012) Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr Cancer Drug Targets 12:197–209
Mellinghoff IK, Cloughesy TF, Mischel PS (2007) PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res 13:378–381. https://doi.org/10.1158/1078-0432.CCR-06-1992
Tomas A, Futter CE, Eden ER (2014) EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 24:26–34. https://doi.org/10.1016/j.tcb.2013.11.002
Kim HJ, Taylor LJ, Bar-Sagi D (2007) Spatial regulation of EGFR signaling by Sprouty2. Curr Biol 17:455–461. https://doi.org/10.1016/j.cub.2007.01.059
Sousa LP, Lax I, Shen H et al (2012) Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc Natl Acad Sci U S A 109:4419–4424. https://doi.org/10.1073/pnas.1200164109
Sigismund S, Avanzato D, Lanzetti L (2018) Emerging functions of the EGFR in cancer. Mol Oncol 12:3–20. https://doi.org/10.1002/1878-0261.12155
Wong ESM, Fong CW, Lim J et al (2002) Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J 21:4796–4808. https://doi.org/10.1093/emboj/cdf493
Walsh AM, Lazzara MJ (2013) Regulation of EGFR trafficking and cell signaling by Sprouty2 and MIG6 in lung cancer cells. J Cell Sci 126:4339–4348. https://doi.org/10.1242/jcs.123208
Zhou X, Xie S, Wu S et al (2017) Golgi phosphoprotein 3 promotes glioma progression via inhibiting Rab5-mediated endocytosis and degradation of epidermal growth factor receptor. Neuro Oncol 19:1628–1639. https://doi.org/10.1093/neuonc/nox104
Wu S, Fu J, Dong Y et al (2018) GOLPH3 promotes glioma progression via facilitating JAK2–STAT3 pathway activation. J Neurooncol 139:269–279. https://doi.org/10.1007/s11060-018-2884-7
Park J-W, Wollmann G, Urbiola C et al (2018) Sprouty2 enhances the tumorigenic potential of glioblastoma cells. Neuro Oncol 20:1044–1054. https://doi.org/10.1093/neuonc/noy028
Walsh AM, Kapoor GS, Buonato JM et al (2015) Sprouty2 drives drug resistance and proliferation in glioblastoma. Mol Cancer Res 13:1227–1237. https://doi.org/10.1158/1541-7786.MCR-14-0183-T
Al-Akhrass H, Naves T, Vincent F et al (2017) Sortilin limits EGFR signaling by promoting its internalization in lung cancer. Nat Commun 8:1182. https://doi.org/10.1038/s41467-017-01172-5
Wilson CM, Naves T, Vincent F et al (2014) Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J Cell Sci 127:3983–3997. https://doi.org/10.1242/jcs.149336
Kondapalli KC, Llongueras JP, Capilla-González V et al (2015) A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat Commun 6:6289. https://doi.org/10.1038/ncomms7289
Gomez Zubieta DM, Hamood MA, Beydoun R et al (2017) MicroRNA-135a regulates NHE9 to inhibit proliferation and migration of glioblastoma cells. Cell Commun Signal 15:55. https://doi.org/10.1186/s12964-017-0209-7
Zhang X, Pickin KA, Bose R et al (2007) Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature 450:741–744. https://doi.org/10.1038/nature05998
Ferby I, Reschke M, Kudlacek O et al (2006) Mig6 is a negative regulator of EGF receptor–mediated skin morphogenesis and tumor formation. Nat Med 12:568–573. https://doi.org/10.1038/nm1401
Ying H, Zheng H, Scott K et al (2010) Mig-6 controls EGFR trafficking and suppresses gliomagenesis. Proc Natl Acad Sci U S A 107:6912–6917. https://doi.org/10.1073/pnas.0914930107
Kim J, Zhang Y, Skalski M et al (2014) microRNA-148a is a prognostic oncomiR that targets MIG6 and BIM to regulate EGFR and apoptosis in glioblastoma. Cancer Res 74:1541–1553. https://doi.org/10.1158/0008-5472.CAN-13-1449
Zaidel-Bar R, ItzkovitzMa’ayan SA et al (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9:858–867. https://doi.org/10.1038/ncb0807-858
Ivaska J, Heino J (2011) Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol 27:291–320. https://doi.org/10.1146/annurev-cellbio-092910-154017
Cruz da Silva E, Dontenwill M, Choulier L, Lehmann M (2019) Role of integrins in resistance to therapies targeting growth factor receptors in cancer. Cancers. https://doi.org/10.3390/cancers11050692
Seguin L, Kato S, Franovic A et al (2014) An integrin β3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol 16:457–468. https://doi.org/10.1038/ncb2953
Barrow-McGee R, Kishi N, Joffre C et al (2016) Beta 1-integrin-c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat Commun 7:11942. https://doi.org/10.1038/ncomms11942
Caswell PT, Chan M, Lindsay AJ et al (2008a) Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 183:143–155. https://doi.org/10.1083/jcb.200804140
Collinet C, Stöter M, Bradshaw CR et al (2010) Systems survey of endocytosis by multiparametric image analysis. Nature 464:243–249. https://doi.org/10.1038/nature08779
Schaffner F, Ray AM, Dontenwill M (2013) Integrin α5β1, the fibronectin receptor, as a pertinent therapeutic target in solid tumors. Cancers 5:27–47. https://doi.org/10.3390/cancers5010027
Janouskova H, Maglott A, Leger DY et al (2012) Integrin α5β1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res 72:3463–3470. https://doi.org/10.1158/0008-5472.CAN-11-4199
Renner G, Janouskova H, Noulet F et al (2016) Integrin α5β1 and p53 convergent pathways in the control of anti-apoptotic proteins PEA-15 and survivin in high-grade glioma. Cell Death Differ 23:640–653. https://doi.org/10.1038/cdd.2015.131
Maglott A, Bartik P, Cosgun S et al (2006) The small alpha5beta1 integrin antagonist, SJ749, reduces proliferation and clonogenicity of human astrocytoma cells. Cancer Res 66:6002–6007. https://doi.org/10.1158/0008-5472.CAN-05-4105
Wang X, Wang Z, Zhang Y et al (2019) Golgi phosphoprotein 3 sensitizes the tumour suppression effect of gefitinib on gliomas. Cell Prolif 52:e12636. https://doi.org/10.1111/cpr.12636
Li Z-X, Qu L-Y, Wen H et al (2014) Mig-6 overcomes gefitinib resistance by inhibiting EGFR/ERK pathway in non-small cell lung cancer cell lines. Int J Clin Exp Pathol 7:7304–7311
Tan X, Thapa N, Sun Y, Anderson RA (2015) A kinase-independent role for EGF receptor in autophagy initiation. Cell 160:145–160. https://doi.org/10.1016/j.cell.2014.12.006
Jo U, Park KH, Whang YM et al (2014) EGFR endocytosis is a novel therapeutic target in lung cancer with wild-type EGFR. Oncotarget 5:1265–1278
Nishimura Y, Bereczky B, Ono M (2007) The EGFR inhibitor gefitinib suppresses ligand-stimulated endocytosis of EGFR via the early/late endocytic pathway in non-small cell lung cancer cell lines. Histochem Cell Biol 127:541–553. https://doi.org/10.1007/s00418-007-0281-y
Blandin A-F, Noulet F, Renner G et al (2016) Glioma cell dispersion is driven by α5 integrin-mediated cell–matrix and cell–cell interactions. Cancer Lett 376:328–338. https://doi.org/10.1016/j.canlet.2016.04.007
Bolte S, Cordelières FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232. https://doi.org/10.1111/j.1365-2818.2006.01706.x
Glushonkov O, Réal E, Boutant E et al (2018) Optimized protocol for combined PALM-dSTORM imaging. Sci Rep. https://doi.org/10.1038/s41598-018-27059-z
Meijering E, Dzyubachyk O, Smal I (2012) Methods for cell and particle tracking. In: Methods in enzymology. Elsevier, pp 183–200
Thuault S, Hayashi S, Lagirand-Cantaloube J et al (2013) P-cadherin is a direct PAX3–FOXO1A target involved in alveolar rhabdomyosarcoma aggressiveness. Oncogene 32:1876–1887. https://doi.org/10.1038/onc.2012.217
Tan X, Lambert PF, Rapraeger AC, Anderson RA (2016) Stress-induced EGFR trafficking: mechanisms, functions, and therapeutic implications. Trends Cell Biol 26:352–366. https://doi.org/10.1016/j.tcb.2015.12.006
Caswell PT, Chan M, Lindsay AJ et al (2008b) Rab-coupling protein coordinates recycling of α5β1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 183:143–155. https://doi.org/10.1083/jcb.200804140
Muller PAJ, Caswell PT, Doyle B et al (2009) Mutant p53 drives invasion by promoting integrin recycling. Cell 139:1327–1341. https://doi.org/10.1016/j.cell.2009.11.026
Caldieri G, Barbieri E, Nappo G et al (2017) Reticulon 3–dependent ER-PM contact sites control EGFR nonclathrin endocytosis. Science 356:617–624. https://doi.org/10.1126/science.aah6152
Zwang Y, Yarden Y (2006) p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J 25:4195–4206. https://doi.org/10.1038/sj.emboj.7601297
Oksvold MP, Huitfeldt HS, Østvold AC, Skarpen E (2002) UV induces tyrosine kinase-independent internalisation and endosome arrest of the EGF receptor. J Cell Sci 115:793–803
Tomas A, Vaughan SO, Burgoyne T et al (2015) WASH and Tsg101/ALIX-dependent diversion of stress-internalized EGFR from the canonical endocytic pathway. Nat Commun. https://doi.org/10.1038/ncomms8324
Cao X, Zhu H, Ali-Osman F, Lo H-W (2011) EGFR and EGFRvIII undergo stress- and EGFR kinase inhibitor-induced mitochondrial translocalization: a potential mechanism of EGFR-driven antagonism of apoptosis. Mol Cancer 10:26. https://doi.org/10.1186/1476-4598-10-26
Cavalli V, Vilbois F, Corti M et al (2001) The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell 7:421–432. https://doi.org/10.1016/S1097-2765(01)00189-7
Macé G, Miaczynska M, Zerial M, Nebreda AR (2005) Phosphorylation of EEA1 by p38 MAP kinase regulates μ opioid receptor endocytosis. EMBO J 24:3235–3246. https://doi.org/10.1038/sj.emboj.7600799
Chen X, Wang Z (2001) Regulation of epidermal growth factor receptor endocytosis by wortmannin through activation of Rab5 rather than inhibition of phosphatidylinositol 3-kinase. EMBO Rep 2:842–849. https://doi.org/10.1093/embo-reports/kve179
Nadanaciva S, Lu S, Gebhard DF et al (2011) A high content screening assay for identifying lysosomotropic compounds. Toxicol Vitro 25:715–723. https://doi.org/10.1016/j.tiv.2010.12.010
Kazmi F, Hensley T, Pope C et al (2013) Lysosomal sequestration (Trapping) of lipophilic amine (Cationic Amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 Cells). Drug Metab Dispos 41:897–905. https://doi.org/10.1124/dmd.112.050054
Englinger B, Kallus S, Senkiv J et al (2018) Lysosomal sequestration impairs the activity of the preclinical FGFR inhibitor PD173074. Cells. https://doi.org/10.3390/cells7120259
Zhitomirsky B, Assaraf YG (2014) Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget 6:1143–1156
Gotink KJ, Broxterman HJ, Labots M et al (2011) Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res 17:7337–7346. https://doi.org/10.1158/1078-0432.CCR-11-1667
Ruzickova E, Skoupa N, Dolezel P et al (2019) The lysosomal sequestration of tyrosine kinase inhibitors and drug resistance. Biomolecules. https://doi.org/10.3390/biom9110675
Li W, Wang H, Yang Y et al (2018) Integrative analysis of proteome and ubiquitylome reveals unique features of lysosomal and endocytic pathways in gefitinib-resistant non-small cell lung cancer cells. Proteomics 18:1700388. https://doi.org/10.1002/pmic.201700388
Lin A, Giuliano CJ, Palladino A et al (2019) Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci Transl Med 11:eaaw8412. https://doi.org/10.1126/scitranslmed.aaw8412
Acknowledgements
This research was funded by Ligue Contre le Cancer, Région Grand-Est, programme-inter region (No S17R417B). Elisabete Cruz Da Silva and Anne-Florence Blandin were PhD students funded by the University of Strasbourg. Marie-Cécile Mercier was a pharmacy intern funded by ARS Grand Est (regional health agency). We thank Romain Vauchelles and the PIQ platform (IBiSa Quest imaging facility) for their assistance in image quantification.
Author information
Authors and Affiliations
Contributions
Participated in research design: ML, MD, LC, PD. Conducted experiments: AFB, ECS, MCM, OG, NES, SD, CS, JD. Performed data analysis: AFB, ECS, MCM, SD, CS, JD. Wrote or contributed to the writing of the manuscript: AFB, ECS, SD, LC, ML. All of the authors reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors disclose no conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2020_3686_MOESM1_ESM.pptx
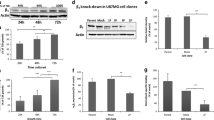
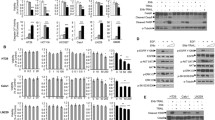
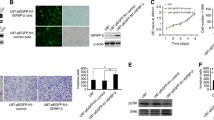
Supplementary file1Supplemental Figure 1: Gefitinib provokes EGFR endocytosis in GBM cells. (A) Immunodetection of EGFR (red) and the endosomal marker EEA1 (green) after 4h treatment with DMSO (control) or gefinitib in LN443 and T98G GBM cells. Magnified images are from the inserts to the peri-nuclear area. Scale bar = 20 μm. (B) Quantification of EGFR/EEA1 colocalization following gefitinib treatment from 10–12 images (3 independent experiments). ***p < 0.001. (C-D) Endocytosis assays of EGF-Alexa488 was performed on LN443, T98G and LNZ308 cells during 1h in presence of gefitinib (20µM). The internalization was measured by integrating fluorescence density of 20-30 cells from 3 independent experiments. ****p < 0.0001. (E) Immunoblot showing α5 integrin and EGFR expression in the 4 cell lines used in this study. Supplemental Figure 2: Gefitinib provokes integrin re-localization in early endosomes. (A) Fluorescence microscopy images of U87 cells treated with gefitinib showing peri-nuclear co-localization of the β1 integrin (cyan) and the early-endosome marker Rab5 (red). (B) The Pearson correlation and Mender’s coefficient were used to quantify the degree of colocalization between the β1 integrin and Rab5. ***p < 0.001. Supplemental Figure 3: Second and third-generation TKIs also induce co-internalization of β1 integrin and EGFR during U87 GBM cell evasion. (A) Confocal images of U87 cells treated with vehicle (control) or TKIs gefitinib (20 µM), afatinib (5 µM), erlotinib (10 µM), dacomitinib (10 µM) or lapatinib (10 µM). Images are representative of 3 independent experiments. High-magnification images are from the inserts into the peri-nuclear area. Scale bar = 20 μm. (B) Quantification of the number of evading cells from U87 and U87α5- treated spheroids. Spheroids were incubated for 24 hours in the presence of DMSO or different TKIs (erlotinib, dacomitinib, lapatinib and afatinib) at the indicated concentrations. Nuclei were stained with DAPI and the number of evading cells was quantified using an ImageJ homemade plugin. Mean of 15 spheroids from 3 independent experiments. **p < 0.05, ***p < 0.001. Supplemental Figure 4: Gefitinib treatment provokes integrin/EGFR relocalization in endosomal compartments of GBM cell lines. Confocal images showing the intracellular distribution (perinuclear region enriched in endomembrane) of EGFR and β1 integrin in LN443, T98G and LNZ308 gefitinib-treated cells. Supplemental Figure 5: Gefitinib treatment does not affect the expression of total EGFR in U87 cells. Left panel: A) Protein expression of EGFR and α5 integrin in U87 GBM cells and U87α5- cells after 24h treatment with DMSO (-) or gefitinib 20µM (+). GADPH was used as loading control. Right panel: Histogram showing the quantification of GADPH-normalized EGFR level of 3 independent experiments. Data represented are the mean +/- s.e.m. B) Quantification of the ratio integrin/EGFR colocalized pixels in the perinuclear compartments of U87 or U87α5- cells that migrated at distance from spheroids after 24 hours of incubation in presence of 20µM gefitinib or DMSO (control). The degree of colocalization between the β1 integrin and EGFR was quantified using an home-made plugin with the ImageJ software. Data expressed as box and whiskers are from at least 30 cells from 10 different fields. Supplemental Figure 6: α5 expression does not affect U87 cell sensitivity to gefitinib in cell growth and cell survival experiments. (A) 2D growth curve of U87 and U87α5- cells in serum-containing medium. (B) Gefitinib dose-response curve on cells growth in 2D after 3 days of treatment. (C) Left panel: phase contrast images of spheroids after 8 days of treatment with indicated concentration of gefitinib. Right panel: dose-response curves of gefitinib on spheroid growth. D) Clonogenic assay in soft agar comparing U87 and U87α5- cell survival in presence of the indicated concentrations of gefitinib. (PPTX 20689 kb)
Rights and permissions
About this article
Cite this article
Blandin, AF., Cruz Da Silva, E., Mercier, MC. et al. Gefitinib induces EGFR and α5β1 integrin co-endocytosis in glioblastoma cells. Cell. Mol. Life Sci. 78, 2949–2962 (2021). https://doi.org/10.1007/s00018-020-03686-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03686-6