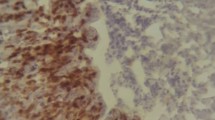
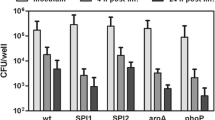
Abstract
Salmonella Enteritidis causes infections in humans and animals which are often associated with extensive gut colonization and bacterial shedding in faeces. The natural presence of flagella in Salmonella enterica has been shown to be enough to induce pro-inflammatory responses in the gut, resulting in recruitment of polymorphonuclear cells, gut inflammation and, consequently, reducing the severity of systemic infection in chickens. On the other hand, the absence of flagellin in some Salmonella strains favours systemic infection as a result of the poor intestinal inflammatory responses elicited. The hypothesis that higher production of flagellin by certain Salmonella enterica strains could lead to an even more immunogenic and less pathogenic strain for chickens was here investigated. In the present study, a Salmonella Enteritidis mutant strain harbouring deletions in clpP and fliD genes (SE ΔclpPfliD), which lead to overexpression of flagellin, was generated, and its immunogenicity and pathogenicity were comparatively assessed to the wild type in chickens. Our results showed that SE ΔclpPfliD elicited more intense immune responses in the gut during early stages of infection than the wild type did, and that this correlated with earlier intestinal and systemic clearance of the bacterium.





Similar content being viewed by others
References
Issenhuth-Jeanjean S, Roggentin P, Mikoleit M, Guibourdenche M, Pinna E, Nair S, Fields PI, Weill F (2014) Supplement 2008-2010 (no. 48) to the White-Kauffmann-Le minor scheme. Res Microbiol 165:526–530. https://doi.org/10.1016/j.resmic.2014.07.004
Spöring I, Felgner S, Preube M, Eckweiler D, Rohde M, Häussler S, Weiss S, Erhardt M (2018) Regulation of flagellum biosynthesis in response to cell envelope stress in Salmonella enterica serovar Typhimurium. mBio 9:e00736–e00717. https://doi.org/10.1128/mBio.00736-17
Chaban B, Hughes HV, Beeby M (2015) The flagellum in bacterial pathogens: for motility and a whole lot more. Semin Cell Dev Biol 46:91–103. https://doi.org/10.1016/j.semcdb.2015.10.032
Santos RL (2014) Pathobiology of Salmonella, intestinal microbiota, and the host innate immune response. Front Immunol 5:252–259. https://doi.org/10.3389/fimmu.2014.00252
Hajam IA, Dar PA, Shahnawaz I, JaumE JC, Lee JH (2017) Bacterial flagellin-a potent immunomodulatory agent. Exp Mol Med 49:147–162. https://doi.org/10.1038/emm.2017.172
Iqbal M, Philbin VJ, Withanage GSK, Wigley P, Beal RK, Goodchild MJ, Barrow P, Mcconell I, Maskell DJ, Young J, Bumstead N, Boyd N, Smith AL (2005) Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar typhimurium identification and functional characterization of chicken toll-like receptor. Infect Immun 73:2344–2350. https://doi.org/10.1128/IAI.73.4.2344
Freitas Neto OC, Setta A, Imre A, Bukovinski A, Elazomi A, Kaiser P, Berchieri A, Junior Barrow P, Jones MA (2013) A flagellated motile Salmonella Gallinarum mutant (SG Fla+) elicits a pro-inflammatory response from avian epithelial cells and macrophages and is less virulent to chickens. Vet Microbiol 165:425–433. https://doi.org/10.1016/j.vetmic.2013.04.015
Renault TT, Abraham AO, Bergmiller T, Paradis G, Rainville S, Charpentier E, Guet CC, Tu Y, Namba K, Keener JP, Minamino T, Erhardt M (2017) Bacterial flagella grow through an injection-diffusion mechanism. Elife 6:1–22. https://doi.org/10.7554/eLife.23136
Albanna A, Sim M, Hoskisson PA, Gillespie C, Rao CV, Aldridge PD (2018) Driving the expression of the Salmonella enterica sv Typhimurium flagellum using flhDC from Escherichia coli results in key regulatory and cellular differences. Sci Rep 8:16705–16716. https://doi.org/10.1038/s41598-018-35005-2
Kato J, Dey S, Soto JE, Butan C, Wilkinson MC, De Guzman RN, Galan JE (2018) A protein secreted by the Salmonella type III secretion system controls needle filament assembly. Elife 7:e35886. https://doi.org/10.7554/eLife.35886
Yonekura K, Maki S, Morgan DG, Derosier DJ, Vonderviszt F, Imada K, Namba K (2000) The bacterial flagellar cap as the rotary promoter of flagellin self-assembly. Science 290:2148–2152. https://doi.org/10.1126/science.290.5499.2148
Tennant SM, Wang J, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM (2011) Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun 79:4175–4185. https://doi.org/10.1128/IAI.05278-11
Tomoyasu T, Ohkishi T, Ukyo Y, Tokumitsu A, Takaya A, Suzuki M, Sekiya K, Kutsukake K, Yamamoto T (2002) The ClpXP ATP-dependent protease regulates flagellum synthesis in Salmonella enterica serovar Typhimurium. J Bacteriol 184:645–653. https://doi.org/10.1128/jb.184.3.645-653.2002
Gast RK, Porter RE Jr (2020) Salmonella infections. In: Swayne et al (eds) Diseases of poultry, 14rd. Wiley, New York, pp 717–753
Barbosa FO, Freitas Neto OC, Batista DFA, Almeida AM, Rubio MDS, Alves LBR, Vasconcelos RO, Barrow PA, Berchieri Junior A Contribution of flagella and motility to gut colonisation and pathogenicity of Salmonella Enteritidis in the chicken. Braz J Microbiol 48:754–759. https://doi.org/10.1016/j.bjm.2017.01.012
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. https://doi.org/10.1073/pnas.120163297
Ye J, Colouris G, Zaretskaya I, Cutcutache I, Rozen S, Maddent L (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform 13:134–145. https://doi.org/10.1186/1471-2105-13-134
Cherepanov PP, Wackernagel W (1995) Gene disruption in Escherichia coli: cR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. https://doi.org/10.1016/0378-1119(95)00193-a
Zancan FB, Berchieri A Jr, Fernandes SA, Gama NMSQ (2000) Salmonella spp. investigation in transport box of day-old birds. Braz J Microbiol 31:230–232. https://doi.org/10.1590/S1517-83822000000300016
Berchieri AJR, Wigley P, Page K, Murphy CK, Barrow PA (2001) Further studies on vertical transmission and persistence of Salmonella enterica serovar Enteritidis phage type 4 in chickens. Avian Pathol 30:297–310. https://doi.org/10.1080/03079450120066304
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250. https://doi.org/10.1158/0008-5472.CAN-04-0496
Fan WQ, Wang HN, Zhang Y, Guan ZB, Wang T, Xu CW, Zhang AY, Yang X (2012) Comparative dynamic distribution of avian infectious bronchitis virus M41, H120, and SAIBK strains by quantitative real-time RT-PCR in SPF chickens. Biosci Biotechnol Biochem 76:2255–2260
De Boever S, Vangestel C, De Backer P, Croubels S, Sys SU (2008) Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet Immunol Immunopathol 122:312–317. https://doi.org/10.1016/j.vetimm.2007.12.002
Setta A, Barrow PA, Kaiser P, Jones MA (2012) Immune dynamics following infection of avian macrophages and epithelial cells with typhoidal and non-typhoidal Salmonella enterica serovars; bacterial invasion and persistence, nitric oxide and oxygen production, differential host gene expression, NF-κB signalling and cell cytotoxicity. Vet Immunol Immunopathol 146:212–224. https://doi.org/10.1016/j.vetimm.2012.03.008
Truong AD, Park B, Ban J, Hong YH (2016) The novel chicken interleukin 26 protein is overexpressed in T cells and induces proinflammatory cytokines. Vet Res 47:65–75. https://doi.org/10.1186/s13567-016-0342-0
Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I (2011) Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis. Infect Immun 79:2755–2763. https://doi.org/10.1128/IAI.01375-10
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Sim M, Koirala S, Picton D, Strahl H, Hoskisson PA, Rao CV, Gillespie CS, Aldridge PD (2017) Growth rate control of flagellar assembly in Escherichia coli strain RP437. Sci Rep 7:1–11. https://doi.org/10.1038/srep41189
Guebel DV, Torres NV (2018) Influence of glucose availability and CRP acetylation on the genome-wide transcriptional response of Escherichia coli: assessment by an optimized factorial microarray analysis. Front Microbiol 9:941–960. https://doi.org/10.3389/fmicb.2018.00941
Yang X, Thornburg T, Suo Z, Jun S, Robison A, Li J, Lim T, Cao L, Hoyt T, Avci R, Pascual DW (2012) Flagella overexpression attenuates Salmonella pathogenesis. PLoS One 7:e46828. https://doi.org/10.1371/journal.pone.0046828
Msadek T, Dartois V, Kunst F, Herbaud ML, Denizot F, Rapoport G (1998) ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol Microbiol 27:899–914. https://doi.org/10.1046/j.1365-2958.1998.00735.x
Knudsen GM, Nielsen M, Thomsen LE, Aabo S, Rychlik I, Olsen JE (2014) The role of ClpP, RpoS and CsrA in growth and filament formation of Salmonella enterica serovar Typhimurium at low temperature. BMC Microbiol 14:208–219. https://doi.org/10.1186/s12866-014-0208-4
Knudsen GM, Olsen JE, Aabo S, Barrow P, Rychlik I, Thomsen LE (2013) ClpP deletion causes attenuation of Salmonella Typhimurium through mis-regulation of RpoS and indirect control of CsrA and the SPI genes. Microbiol 159:1497–1509. https://doi.org/10.1099/mic.0.065797-0
Allen-vercoe E, Woodward MJ (1999) The role of flagella, but not fimbriae, in the adherence of Salmonella enterica serotype Enteritidis to chick gut explant. J Med Microbiol 48:771–780. https://doi.org/10.1099/00222615-48-8-771
Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P (2009) The immunobiology of avian systemic salmonellosis. Vet Immunol Immunopathol 128:53–59. https://doi.org/10.1016/j.vetimm.2008.10.295
Kurtz JR, Goggins JL, McLachlan (2017) Salmonella infection: interplay between the bacteria and host immune system. Immunol Lett 190:42–40. https://doi.org/10.1016/j.imlet.2017.07.006
Sanders CJ, Franchi L, Yarovinsky F, Uematsu S, Núñez G, Gewirtz AT (2009) Induction of adaptive immunity by flagellin does not require robust activation of innate immunity. Eur J Immunol 39:359–371. https://doi.org/10.1002/eji.200838804
Paul MS, Brisbin JT, Abdul-Careem MF, Sharif S (2013) Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet Immunol Immunopathol 152:191–199. https://doi.org/10.1016/j.vetimm.2012.10.013
Kremer CJ, O’Meara KM, Layton SL, Hargis BM, Cole K (2011) Evaluation of recombinant Salmonella expressing the flagellar protein fliC for persistence and enhanced antibody response in commercial turkeys. Poult Sci 90:752–758. https://doi.org/10.3382/ps.2010-01076
Kaiser P, Stäheli P (2014) Avian cytokines and chemokines. In: Avian immunology, 2nd edn. Elsevier, Amsterdam, pp 189–204. https://doi.org/10.1016/B978-0-12-396965-1.00010-8
Lee SJ, Benoun J, Sheridan BS, Fogassy Z, Pham O, Pham QM, Puddignton L, Mcsorley SJ (2017) Dual immunization with SseB/flagellin provides enhanced protection against Salmonella infection mediated by circulating memory cells. J Immunol 199:1353–1361. https://doi.org/10.4049/jimmunol.1601357
Funding
This work was supported by São Paulo Research Foundation (FAPESP) (grant numbers: 2018/04883-8 (F. O. Barbosa); 2016/10369-0 (A. Berchieri Jr)), Coordination of Improvement of Higher Education Personnel (CAPES), and National Council of Technological and Scientific Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Responsible Editor: Waldir P. Elias
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 33 kb)
Rights and permissions
About this article
Cite this article
de Oliveira Barbosa, F., de Freitas Neto, O.C., Rodrigues Alves, L.B. et al. Immunological and bacteriological shifts associated with a flagellin-hyperproducing Salmonella Enteritidis mutant in chickens. Braz J Microbiol 52, 419–429 (2021). https://doi.org/10.1007/s42770-020-00399-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00399-7