Abstract
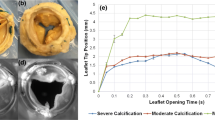
Coronary flow induces hemodynamic alterations in the aortic sinus region. The objectives of this study are to: (1) investigate the differences among sinus hemodynamics and leaflet wall shear stresses engendered by the left versus right versus non-coronary flow and (2) correlate respective wall shear stresses with leaflet calcification in patients. A left heart simulator flow loop with a tunable coronary circuit provided physiological coronary flow waveforms corresponding to the left coronary cusp case (LCC), right coronary cusp case (RCC), and non-coronary cusp case (NCC). High spatio-temporal resolution particle image velocimetry was conducted to quantify leaflet wall shear stress and sinus vorticity fields and to measure aortic leaflet tip kinematics. Thirty-one patients with severe calcific aortic valve disease were segmented from CT data for the calcific volumes in their respective left, right, and non-coronary cusps. Leaflet tip position during systole shows the RCC has a wider leaflet opening compared to LCC and NCC. Velocity and vorticity fields combined with leaflet position data show that sinus vorticity is diminished (peak ~ 43 s−1) in the LCC while RCC and NCC maintain high vorticity (~ 1200 and ~ 950 s−1 respectively). WSS magnitudes greater than 0.3 Pa show 20 and 81% greater occurrences in the LCC and RCC respectively compared to NCC. Significant differences [X2 (2, n = 31) = 7.31, p = 0.0258] between the calcification levels in each cusp of the patient population. Coronary flow differences between LCC, RCC, and NCC show significant impact on leaflet kinematics and sinus flow hemodynamics. Clinical data correlations of the coronary flow cases indicate the left coronary cusp has a higher likelihood of calcification compared to the right.









Similar content being viewed by others
References
Bonow, R. O., and P. Greenland. Population-wide trends in aortic stenosis incidence and outcomes. Circulation 131:969–971, 2015.
Butcher, J., C. A. Simmons, and J. Warnock. Review: mechanobiology of the aortic heart valve. J. Heart Valve Dis. 17:62–73, 2008.
Chiu, J.-J., and S. Chien. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011. https://doi.org/10.1152/physrev.00047.2009.
Foroutan, F., G. H. Guyatt, K. O’Brien, E. Bain, M. Stein, S. Bhagra, D. Sit, R. Kamran, Y. Chang, T. Devji, H. Mir, V. Manja, T. Schofield, R. A. Siemieniuk, T. Agoritsas, R. Bagur, C. M. Otto, and P. O. Vandvik. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ 2016. https://doi.org/10.1136/bmj.i5065.
Freeman, R. V., and C. M. Otto. Spectrum of calcific aortic valve disease. Pathog. Dis. Prog. Treat. Strateg. 111:3316–3326, 2005.
Gould, S. T., S. Srigunapalan, C. A. Simmons, and K. S. Anseth. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ. Res. 113:186–197, 2013.
Hatoum, H., and L. P. Dasi. Spatiotemporal complexity of the aortic sinus vortex as a function of leaflet calcification. Ann. Biomed. Eng. 47(4):1116–1128, 2019.
Hatoum, H., J. Dollery, S. M. Lilly, J. A. Crestanello, and L. P. Dasi. Effect of severe bioprosthetic valve tissue ingrowth and inflow calcification on valve-in-valve performance. Journal of biomechanics 74:171–179, 2018.
Hatoum, H., J. Dollery, S. M. Lilly, J. A. Crestanello, and L. P. Dasi. Implantation depth and rotational orientation effect on valve-in-valve hemodynamics and sinus flow. Ann. Thorac. Surg. 106(1):70–78, 2018.
Hatoum, H., J. Dollery, S. M. Lilly, J. A. Crestanello, and L. P. Dasi. Sinus hemodynamics variation with tilted transcatheter aortic valve deployments. Ann. Biomed. Eng 47(1):75–84, 2019.
Hatoum, H., J. Dollery, S. M. Lilly, J. Crestanello, and L. P. Dasi. Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: an in vitro study. J. Thorac. Cardiovasc. Surg. 157(2):540–549, 2019.
Hatoum, H., J. Dollery, S. M. Lilly, J. A. Crestanello, and L. P. Dasi. Effect of severe bioprosthetic valve tissue ingrowth and inflow calcification on valve-in-valve performance. J. Biomech. 74:171–179, 2018.
Hatoum, H., P. Maureira, S. Lilly, and L. P. Dasi. Impact of leaflet laceration on transcatheter aortic valve-in-valve washout: BASILICA to solve neosinus and sinus stasis. JACC Cardiovasc. Interv. 12(13):1229–1237, 2019.
Hatoum, H., B. L. Moore, P. Maureira, J. Dollery, J. A. Crestanello, and L. P. Dasi. Aortic sinus flow stasis likely in valve-in-valve transcatheter aortic valve implantation. J. Thorac. Cardiovasc. Surg. 154:32–43, 2017.
James, T. N. Anatomy of the coronary arteries in health and disease. Circulation 32:1020–1033, 1965.
Johnson, K., P. Sharma, and J. Oshinski. Coronary artery flow measurement using navigator echo gated phase contrast magnetic resonance velocity mapping at 3.0t. J. Biomech. 41:595–602, 2008.
Khalique, O. K., R. T. Hahn, H. Gada, T. M. Nazif, T. P. Vahl, I. George, B. Kalesan, M. Forster, M. B. Williams, M. B. Leon, A. J. Einstein, T. C. Pulerwitz, Gregory D. N. Pearson, and S. K. Kodali. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Interv 7:885–894, 2014.
Krzanowski, M., W. Bodzoń, and P. P. Dimitrow. Imaging of all three coronary arteries by transthoracic echocardiography. An illustrated guide. Cardiovasc Ultrasound 1:16, 2003.
Lei, M., D. P. Giddens, S. A. Jones, F. Loth, and H. Bassiouny. Pulsatile flow in an end-to-side vascular graft model: comparison of computations with experimental data. J. Biomech. Eng. 123:80–87, 2000.
Leopold, J. A. Cellular mechanisms of aortic valve calcification. Circulation 5:605–614, 2012.
McCollough, C. H., S. Ulzheimer, S. S. Halliburton, K. Shanneik, R. D. White, and W. A. Kalender. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 243(2):527–538, 2007.
Milin, A. C., G. Vorobiof, O. Aksoy, and R. Ardehali. Insights into aortic sclerosis and its relationship with coronary artery disease. J. Am. Heart Assoc. 3:e001111, 2014.
Moore, B., and L. P. Dasi. Spatiotemporal complexity of the aortic sinus vortex. Exp. Fluids 55:1770, 2014.
Moore, B. L., and L. P. Dasi. Coronary flow impacts aortic leaflet mechanics and aortic sinus hemodynamics. Ann. Biomed. Eng. 43:2231–2241, 2015.
Otto, C. M. Calcification of bicuspid aortic valves. Heart 88:321–322, 2002.
Peacock, J. A. An in vitro study of the onset of turbulence in the sinus of valsalva. Circ. Res. 67:448–460, 1990.
Piola, M., R. Vismara, G. Tasca, F. Lucherini, P. Redaelli, M. Soncini, C. Romagnoni, A. Mangini, C. Antona, and G. B. Fiore. Design of a simple coronary impedance simulator for the in vitro study of the complex coronary hemodynamics. Physiol. Meas. 37:2274–2285, 2016.
Sankaran, S., M. E. Moghadam, A. M. Kahn, E. E. Tseng, J. M. Guccione, and A. L. Marsden. Patient-specific multiscale modeling of blood flow for coronary artery bypass graft surgery. Ann. Biomed. Eng. 40:2228–2242, 2012.
Schäfers, H.-J., W. Schmied, G. Marom, and D. Aicher. Cusp height in aortic valves. J. Thorac. Cardiovasc. Surg. 146:269–274, 2013.
Sluysmans, T., and S. D. Colan. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J. Appl. Physiol. 99(2005):445–457, 1985.
Sucosky, P., K. Balachandran, A. Elhammali, H. Jo, and A. P. Yoganathan. Altered shear stress stimulates upregulation of endothelial Vcam-1 and Icam-1 in a Bmp-4- and Tgf-Β1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29:254–260, 2009.
Yap, C. H., N. Saikrishnan, G. Tamilselvan, and A. P. Yoganathan. Experimental measurement of dynamic fluid shear stress on the aortic surface of the aortic valve leaflet. Biomech. Model. Mechanobiol. 11:171–182, 2012.
Acknowledgments
This research was supported by the National Institutes of Health under Award Number 3R01HL118924 and 5R01HL119824 and by the American Heart Association under Award Number.
Conflict of interest
Dr. Dasi reports having a patent application filed on novel polymeric valves. No other conflicts were reported.
Funding
The research done was partly supported by National Institutes of Health (NIH) under Award Number 3R01HL119824 and 5R01HL119824 and by the American Heart Association under award number 19POST34380804.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ender A. Finol oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dorma C. Flemister and Hoda Hatoum shared the authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 17286 kb)
Rights and permissions
About this article
Cite this article
Flemister, D.C., Hatoum, H., Guhan, V. et al. Effect of Left and Right Coronary Flow Waveforms on Aortic Sinus Hemodynamics and Leaflet Shear Stress: Correlation with Calcification Locations. Ann Biomed Eng 48, 2796–2808 (2020). https://doi.org/10.1007/s10439-020-02677-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02677-9