Abstract
Purpose
We have previously demonstrated by MRI that high glucose stimulates efflux of zinc ions from the prostate. To our knowledge, this phenomena had not been reported previously and the mechanism remains unknown. Here, we report some initial observations that provide new insights into zinc processing during glucose-stimulated zinc secretion (GSZS) in the immortalized human prostate epithelial cell line, PNT1A. Additionally, we identified the subtypes of zinc-containing cells in human benign prostatic hyperplasia (BPH) tissue to further identify which cell types are likely responsible for zinc release in vivo.
Procedure
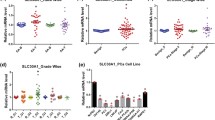
An intracellular fluorescence marker, FluoZin-1-AM, was used to assess the different roles of ZnT1 and ZnT4 in zinc homeostasis in wild type (WT) and mRNA knockdown PNT1A cell lines. Additionally, Bafilomycin A1 (Baf) was used to disrupt lysosomes and assess the role of lysosomal storage during GSZS. ZIMIR, an extracellular zinc-responsive fluorescent marker, was used to assess dynamic zinc efflux of WT and ZnT1 mRNA knockdown cells exposed to high glucose. Electron microscopy was used to assess intracellular zinc storage in response to high glucose and evaluate how Bafilomycin A1 affects zinc trafficking. BPH cells were harvested from transurtheral prostatectomy tissue and stained with fluorescent zinc granule indicator (ZIGIR), an intracellular zinc-responsive fluorescent marker, before being sorted for cell types using flow cytometry.
Results
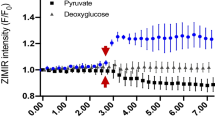
Fluorescent studies demonstrate that ZnT1 is the major zinc efflux transporter in prostate epithelial cells and that loss of ZnT1 via mRNA knockdown combined with lysosomal storage disruption results in a nearly 4-fold increase in cytosolic zinc. Knockdown of ZnT1 dramatically reduces zinc efflux during GSZS. Electron microscopy (EM) reveals that glucose stimulation significantly increases lysosomal storage of zinc; disruption of lysosomes via Baf or ZnT4 mRNA knockdown increases multi-vesicular body (MVB) formation and cytosolic zinc levels. In human BPH tissue, only the luminal epithelial cells contained significant amounts of zinc storage granules.
Conclusions
Exposure of prostate epithelial cells to high glucose alters zinc homeostasis by inducing efflux of zinc ions via ZnT1 channels and increasing lysosomal storage via ZnT4. Given that prostate cancer cells undergo profound metabolic changes that result in reduced levels of total zinc, understanding the complex interplay between glucose exposure and zinc homeostasis in the prostate may provide new insights into the development of prostate carcinogenesis.






Similar content being viewed by others
References
Costello LC, Franklin RB, Feng P (June 2005) Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion 5(3):143–153. https://doi.org/10.1016/j.mito.2005.02.001
Kavanagh JP (1994) Isocitric and citric acid in human prostatic and seminal fluid: implications for prostatic metabolism and secretion. Prostate 24(3):139–142
Singh KK et al (2006) Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol Cancer 5:14. https://doi.org/10.1186/1476-4598-5-14
Kelleher SL et al (2011) Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr 2(2):101–111. https://doi.org/10.3945/an.110.000232
Costello LC, Franklin RB (1998) Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate 35(4):285–296
Franklin RB et al (2005) Zinc and zinc transporters in normal prostate function and the pathogenesis of prostate cancer. Front Biosci 10:2230–2239
Verze P et al (2016) The role of the prostate in male fertility, health and disease. Nat Rev Urol 13(7):379–386. https://doi.org/10.1038/nrurol.2016.89
Jayaraman AK, Jayaraman S (2011) Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the ZnT-1 zinc transporter gene in pancreatic cancer cells. J Nutr Biochem 22(1):79–88. https://doi.org/10.1016/j.jnutbio.2009.12.001
Kukic I et al (2014) Zn2+ Efflux through Lysosomal Exocytosis Prevents Zn2+-Induced Toxicity. J Cell Sci 127:3094–3103. https://doi.org/10.1242/jcs.145318
Takagishi T, et al. (2017) Recent advances in the role of SLC39A/ZIP zinc transporters in vivo. Int J Mol Sci 18: 2708–2729. https://doi.org/10.3390/ijms18122708
Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC (2003) Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J Inorg Biochem 96:435–442. https://doi.org/10.1016/S0162-0134(03)00249-6
Franklin RB et al (2005) HZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 4:32. https://doi.org/10.1186/1476-4598-4-32
Palmiter RD (2004) Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc Nati Acad Sci U S A 101:4918–4923. https://doi.org/10.1073/pnas.0401022101
Sankavaram K, Freake HC (June 2012) The effects of transformation and ZnT-1 silencing on zinc homeostasis in cultured cells. J Nutr Biochem 23(6):629–634. https://doi.org/10.1016/j.jnutbio.2011.03.006
Beck FWJ et al (2004) Differential expression of HZnT-4 in human prostate tissues. Prostate 58(4):374–381. Wiley Online Library. https://doi.org/10.1002/pros.10344
Kolenko V et al (2013) Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol 10(4):219–226. https://doi.org/10.1038/nrurol.2013.43
Henshall SM et al (2003) Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 22(38):6005–6012. https://doi.org/10.1038/sj.onc.1206797
Jordan C, Veronica M et al (2016) Zinc-sensitive MRI contrast agent detects differential release of Zn(II) Ions from the healthy vs. malignant mouse prostate. Proc Natl Acad Sci U S A 113(37):E5464–E5471. https://doi.org/10.1073/pnas.1609450113
Mauvezin C, Neufeld TP (2015) Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 11(8):1437–1438. https://doi.org/10.1080/15548627.2015.1066957
Shin H, Bang S, Kim J, Jun JH, Song H, Lim HJ (2017) The formation of multivesicular bodies in activated blastocysts is influenced by autophagy and FGF signaling in mice. Sci Rep 7:41986. https://doi.org/10.1038/srep41986
Kanemoto S et al (2016) Multivesicular body formation enhancement and exosome release during endoplasmic reticulum stress. Biochem Biophys Res Commun 480(2):166–172. https://doi.org/10.1016/j.bbrc.2016.10.019
Li D et al (2011) Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR). Proc Natl Acad Sci U S A 108(52):21063–21068. https://doi.org/10.1073/pnas.1109773109
Joseph DB, Henry GH, Malewska A, Iqbal NS, Ruetten HM, Turco AE, Abler LL et al (2020) “Urethral luminal epithelia are castration-insensitive progenitors of the proximal prostate.” Preprint. Cell Biol. https://doi.org/10.1101/2020.02.19.937615
Henry GH, Loof N, Strand DW (December 2017) OMIP-040: optimized gating of human prostate cellular subpopulations. Cytometry Part A 91(12):1147–1149. https://doi.org/10.1002/cyto.a.23187
Strand DW, Costa DN, Francis F, Ricke WA, Roehrborn CG (2017) Targeting phenotypic heterogeneity in benign prostatic hyperplasia. Differentiation; Res Biol Divers 96:49–61. https://doi.org/10.1016/j.diff.2017.07.005
Ghazvini Zadeh EH, et al. (2020) ZIGIR, a granule-specific Zn2+ indicator, reveals human islet alpha cell heterogeneity. Cell Rep 32:107904–107919. https://doi.org/10.1016/j.celrep.2020.107904
Cunningham D, You Z (2015) In vitro and in vivo model systems used in prostate cancer research. J Biol Methods 2(1): e17–e31. https://doi.org/10.14440/jbm.2015.63
Kukic I, Lee JK, Coblentz J, Kelleher SL, Kiselyov K (April 15, 2013) Zinc-dependent lysosomal enlargement in TRPML1-deficient cells involves MTF-1 transcription factor and ZnT4 (Slc30a4) transporter. Biochem J 451(2):155–163. https://doi.org/10.1042/BJ20121506
Soekmadji C, Russell PJ, Nelson CC (2013) Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers 5(4):1522–1544. https://doi.org/10.3390/cancers5041522
Shusterman E, Beharier O, Levy S, Zarivach R, Etzion Y, Campbell CR, Lee I-H et al (2017) Zinc transport and the inhibition of the L-type calcium channel are two separable functions of ZnT-1. Metallomics 9(3):228–238. https://doi.org/10.1039/C6MT00296J
Tvedt KE, Kopstad G, Haugen OA, Halgunset J (n.d.) “Subcellular concentrations of calcium, zinc, and magnesium in benign nodular hyperplasia of the human prostate: X-ray microanalysis of freeze-dried cryosections.” Intracellular Calcium 7
Xin L (August 8, 2013) Cells of origin for cancer: an updated view from prostate cancer. Oncogene 32(32):3655–3663. https://doi.org/10.1038/onc.2012.541
Waters KM et al (2011) No association of type-2 diabetes risk variants and prostate cancer risk: the multiethnic cohort and PAGE. Cancer Epidemiol Biomark Prev 20(9):1979–1981. https://doi.org/10.1158/1055-9965.EPI-11-0019
Waters KM et al (2009) Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol 169(8):937–945. https://doi.org/10.1093/aje/kwp003
Breyer BN, Sarma AV (2014) Hyperglycemia and insulin resistance and the risk of BPH/LUTS: an update of recent literature. Curr Urol Rep 15(12):462. https://doi.org/10.1007/s11934-014-0462-x
Sapota A et al (2009) Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions. BioMetals 22(6):1041. Springer Link. https://doi.org/10.1007/s10534-009-9255-y
Acknowledgements
We thank Dr. Wen-hong Li (Department of Cell Biology, UT Southwestern Medical Center) for the gift of the ZIMIR and critical reading of the manuscript.
Funding
This work was supported by grants from the National Institutes of Health (R01-DK095416), the Robert A. Welch Foundation (AT-584), and the Cancer Prevention & Research Institute of Texas (RP-180178).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
A.D.S. is a co-founder of VitalQuan, LLC, which is developing MRI and fluorescence sensors for detecting Zn2+ in tissues.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 313 kb)
Rights and permissions
About this article
Cite this article
Lo, ST., Parrott, D., Jordan, M.V.C. et al. The Roles of ZnT1 and ZnT4 in Glucose-Stimulated Zinc Secretion in Prostate Epithelial Cells. Mol Imaging Biol 23, 230–240 (2021). https://doi.org/10.1007/s11307-020-01557-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01557-x