Abstract
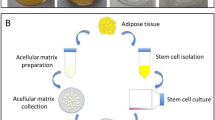
To boost the healing process in a full-thickness wound, a simple and efficient strategy based on adipose-derived mesenchymal stem cells (ADSCs) transplantation is described in this work. To increase the chance of ADSCs immobilization in the wound bed and prevent its migration, these cells are fully grown on the surface of a thermoresponsive dressing membrane under in vitro condition. Then, the cells sheet with their secreted extracellular matrix (ECM) is transferred to the damaged skin with the help of this dressing membrane. This membrane remains on wound bed and acts both as a cell sheet transfer vehicle, after external reduction of temperature, and protect wound during the healing process like a common wound dressing. The visual inspection of wounded skin (rat animal model) at selected time intervals shows a higher wound closure rate for ADSCs treated group. For this group of rats, the better quality of reconstructed tissue is approved by results of histological and immunohistochemical analysis since the higher length of the new epidermis, the higher thickness of re-epithelialization layer, a higher level of neovascularization and capillary density, and the least collagen deposition are detected in the healed tissue.







Similar content being viewed by others
References
Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C. 2015;48:651–62. http://linkinghub.elsevier.com/retrieve/pii/S0928493114008662.
Shams E, Yeganeh H, Naderi-Manesh H, Gharibi R, Mohammad, Hassan Z. Polyurethane/siloxane membranes containing graphene oxide nanoplatelets as antimicrobial wound dressings: in vitro and in vivo evaluations. J Mater Sci Mater Med. 2017;28:75. http://link.springer.com/10.1007/s10856-017-5881-z.
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58:81–94. https://www.karger.com/Article/FullText/454919.
Yari A, Yeganeh H, Bakhshi H, Gharibi R. Preparation and characterization of novel antibacterial castor oil-based polyurethane membranes for wound dressing application. J Biomed Mater Res Part A. 2014;102:84–96. http://doi.wiley.com/10.1002/jbm.a.34672.
Gharibi R, Yeganeh H, Rezapour-Lactoee A, Hassan ZM. Stimulation of wound healing by electroactive, antibacterial, and antioxidant polyurethane/siloxane dressing membranes: in vitro and in vivo evaluations. ACS Appl Mater Interfaces. 2015;7:24296–311. http://pubs.acs.org/doi/10.1021/acsami.5b08376.
Lin Y-J, Lee G-H, Chou C-W, Chen Y-P, Wu T-H, Lin H-R. Stimulation of wound healing by PU/hydrogel composites containing fibroblast growth factor-2. J Mater Chem B. 2015;3:1931–41.
Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds. 2005;4:23–44.
Kasuya A, Tokura Y. Attempts to accelerate wound healing. J Dermatol Sci. 2014;76:169–72. http://linkinghub.elsevier.com/retrieve/pii/S0923181114002540.
Loss M, Wedler V, Künzi W, Meuli-Simmen C, Meyer V. Artificial skin, split-thickness autograft and cultured autologous keratinocytes combined to treat a severe burn injury of 93% of TBSA. Burns. 2000;26:644–52. http://linkinghub.elsevier.com/retrieve/pii/S0305417900000450.
Kato Y, Iwata T, Morikawa S, Yamato M, Okano T, Uchigata Y. Allogeneic transplantation of an adipose-derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes. 2015;64:2723–34. http://diabetes.diabetesjournals.org/content/early/2015/03/18/db14-1133.abstract.
Metcalfe AD, Ferguson MW. Skin stem and progenitor cells: using regeneration as a tissue-engineering strategy. Cell Mol Life Sci. 2008;65:24–32. https://www.ncbi.nlm.nih.gov/pubmed/18030423.
Dieckmann C, Renner R, Milkova L, Simon JC. Regenerative medicine in dermatology: biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp Dermatol. 2010;19:697–706. https://www.ncbi.nlm.nih.gov/pubmed/20545761.
Ribeiro J, Pereira T, Amorim I, Caseiro AR, Lopes MA, Lima J. et al. Cell therapy with human MSCs isolated from the umbilical cord Wharton jelly associated to a PVA membrane in the treatment of chronic skin wounds. Int J Med Sci. 2014;11:979–87. https://www.ncbi.nlm.nih.gov/pubmed/25076843.
Li A, Pouliot N, Redvers R, Kaur P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J Clin Invest. 2004;113:390–400.
Gadelkarim M, Abushouk AI, Ghanem E, Hamaad AM, Saad AM, Abdel-Daim MM. Adipose-derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Biomed Pharmacother. 2018;107:625–33. https://linkinghub.elsevier.com/retrieve/pii/S0753332218322388.
Hamada M, Iwata T, Kato Y, Washio K, Morikawa S, Sakurai H. et al. Xenogeneic transplantation of human adipose-derived stem cell sheets accelerate angiogenesis and the healing of skin wounds in a Zucker Diabetic Fatty rat model of obese diabetes. Regen. Ther. 2017;6:65–73. http://linkinghub.elsevier.com/retrieve/pii/S2352320416300724.
Isakson M, de Blacam C, Whelan D, McArdle A, Clover AJP. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cells Int. 2015;2015:1–12. https://www.ncbi.nlm.nih.gov/pubmed/26106431.
Teng M, Huang Y, Zhang H. Application of stems cells in wound healing–an update. Wound Repair Regen. 2014;22:151–60. https://www.ncbi.nlm.nih.gov/pubmed/24635168.
Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp NP. et al. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784–95. https://www.ncbi.nlm.nih.gov/pubmed/18951271.
McCarthy ME, Brown TA, Bukowska J, Bunnell BA, Frazier T, Wu X. et al. Therapeutic applications for adipose-derived stem cells in wound healing and tissue engineering. Curr Stem Cell Rep. 2018;4:127–37. http://link.springer.com/10.1007/s40778-018-0125-9.
Fromm-Dornieden C, Koenen P. Adipose derived stem cells (ASC) in wound healing: recent results in vitro and in vivo. OA Mol Cell Biol. 2013;1:1–6. http://www.oapublishinglondon.com/article/1147.
Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22:313–25. https://www.ncbi.nlm.nih.gov/pubmed/24844331.
Kim WS, Park BS, Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9:879–87. https://www.ncbi.nlm.nih.gov/pubmed/19522555.
Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev. 2008;60:277–85. https://www.ncbi.nlm.nih.gov/pubmed/18006178.
Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M. et al. Reconstruction of functional tissues with cell sheet engineering. Biomater. 2007;28:5033–43. https://www.ncbi.nlm.nih.gov/pubmed/17761277.
Matsuda N, Shimizu T, Yamato M, Okano T. Tissue engineering based on cell sheet technology. Adv Mater. 2007;19:3089–99.
Cerqueira MT, Pirraco RP, Santos TC, Rodrigues DB, Frias AM, Martins AR. et al. Human adipose stem cells cell sheet constructs impact epidermal morphogenesis in full-thickness excisional wounds. Biomacromolecules. 2013;14:3997–4008. https://www.ncbi.nlm.nih.gov/pubmed/24093541.
Lin YC, Grahovac T, Oh SJ, Ieraci M, Rubin JP, Marra KG. Evaluation of a multi-layer adipose-derived stem cell sheet in a full-thickness wound healing model. Acta Biomater. 2013;9:5243–50. https://www.ncbi.nlm.nih.gov/pubmed/23022891.
Chua AWC, Ma DR, Song IC, Phan TT, Lee ST, Song C. In vitro evaluation of fibrin mat and TegadermTM wound dressing for the delivery of keratinocytes—implications of their use to treat burns. Burns. 2008;34:175–80. http://linkinghub.elsevier.com/retrieve/pii/S0305417907001957.
Chong E, Phan T, Lim I, Zhang Y, Bay B, Ramakrishna S. et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3:321–30. https://linkinghub.elsevier.com/retrieve/pii/S1742706107000153.
Rezapour-Lactoee A, Yeganeh H, Ostad SN, Gharibi R, Mazaheri Z, Ai J. Thermoresponsive polyurethane/siloxane membrane for wound dressing and cell sheet transplantation: in-vitro and in-vivo studies. Mater Sci Eng C. 2016;69:804–14. http://linkinghub.elsevier.com/retrieve/pii/S0928493116307408.
Yu J, Wang M-Y, Tai H-C, Cheng N-C. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018;77:191–200. https://doi.org/10.1016/j.actbio.2018.07.022.
Eke G, Mangir N, Hasirci N, MacNeil S, Hasirci V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomater. 2017;129:188–98. https://doi.org/10.1016/j.biomaterials.2017.03.021.
Silva AKA, Perretta S, Perrod G, Pidial L, Lindner V, Carn F. et al. Thermoresponsive gel embedded with adipose stem-cell-derived extracellular vesicles promotes esophageal fistula healing in a thermo-actuated delivery strategy. ACS Nano. 2018;12:9800–14. http://pubs.acs.org/doi/10.1021/acsnano.8b00117.
Sasagawa T, Shimizu T, Sekiya S, Yamato M, Okano T. Comparison of angiogenic potential between prevascular and non-prevascular layered adipose-derived stem cell-sheets in early post-transplanted period. J Biomed Mater Res A. 2014;102:358–65. https://www.ncbi.nlm.nih.gov/pubmed/23533096.
Yu J, Tu YK, Tang YB, Cheng NC. Stemness and transdifferentiation of adipose-derived stem cells using L-ascorbic acid 2-phosphate-induced cell sheet formation. Biomaterials. 2014;35:3516–26.
Hardwicke JT, Hart J, Bell A, Duncan R, Thomas DW, Moseley R. The effect of dextrin-rhEGF on the healing of full-thickness, excisional wounds in the (db/db) diabetic mouse. J Control Release. 2011;152:411–7.
Milan PB, Lotfibakhshaiesh N, Joghataie MT, Ai J, Pazouki A, Kaplan DL, et al. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016;45:234–46.
Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–52.
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59.
Wong VW, Gurtner GC. Tissue engineering for the management of chronic wounds: current concepts and future perspectives. Exp Dermatol. 2012;21:729–34.
Maharlooei MK, Bagheri M, Solhjou Z, Jahromi BM, Akrami M, Rohani L, et al. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pr. 2011;93:228–34.
Song SY, Jung JE, Jeon YR, Tark KC, Lew DH. Determination of adipose-derived stem cell application on photo-aged fibroblasts, based on paracrine function. Cytotherapy. 2011;13:378–84.
Park BS, Jang KA, Sung JH, Park JS, Kwon YH, Kim KJ, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg. 2008;34:1323–6.
Kim WS, Park BS, Sung JH. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch Dermatol Res. 2009;301:329–36.
Moon KM, Park YH, Lee JS, Chae YB, Kim MM, Kim DS, et al. The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int J Mol Sci. 2012;13:1239–57.
Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol. 2012;24:136–43. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3346902/.
Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316:48–54.
Yan Y, Liu Y, Liu D, He L, Guan L, Wang Y, et al. Differentiation of adipose-derived adult stem cells into epithelial-like stem cells. Ann Anat. 2013;195:212–8.
Lacroix S, Bouez C, Vidal S, Cenizo V, Reymermier C, Justin V, et al. Supplementation with a complex of active nutrients improved dermal and epidermal characteristics in skin equivalents generated from fibroblasts from young or aged donors. Biogerontology. 2007;8:97–109.
Ventayol M, Vinas JL, Sola A, Jung M, Brune B, Pi F, et al. miRNA let-7e targeting MMP9 is involved in adipose-derived stem cell differentiation toward epithelia. Cell Death Dis. 2014;5:e1048.
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–25.
Rodrigues C, de Assis AM, Moura DJ, Halmenschlager G, Saffi J, Xavier LL, et al. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS ONE. 2014;9:e96241.
Li X, Hamada T, Ohata C, Furumura M, Hashimoto T. Potential mesenchymal stem cell therapy for skin diseases. Exp Dermatol. 2013;22:515–6.
Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–47.
Zhou K, Ma Y, Brogan MS. Chronic and non-healing wounds: the story of vascular endothelial growth factor. Med Hypotheses. 2015;85:399–404.
Nomi M, Atala A, Coppi PD, Soker S. Principals of neovascularization for tissue engineering. Mol Asp Med. 2002;23:463–83.
Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, Andre M, Cousin B, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arter Thromb Vasc Biol. 2009;29:503–10.
Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–53.
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rezapour-Lactoee, A., Yeganeh, H., Gharibi, R. et al. Enhanced healing of a full-thickness wound by a thermoresponsive dressing utilized for simultaneous transfer and protection of adipose-derived mesenchymal stem cells sheet. J Mater Sci: Mater Med 31, 101 (2020). https://doi.org/10.1007/s10856-020-06433-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06433-2