Abstract
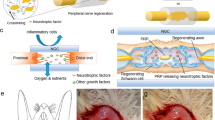
The objective of this paper is to investigate the possibility and efficacy of recurrent laryngeal nerve repair by transplantation of co-cultured Schwann cells and neural stem cells (NSCs) in laminin-chitosan-poly-lactic-co-glycolic acid (laminin-chitosan-PLGA) nerve conduits in rats. A laminin-chitosan-PLGA conduit was used in a rat recurrent laryngeal nerve transection model. The rat recurrent laryngeal nerve was dissected to generate a 5 mm defect. Then, a laminin-chitosan-PLGA nerve conduit with or without Schwann cells and NSCs in the lumen was transplanted into the defect. A total of 96 female rats were randomised into six groups: co-culture of NSCs and Schwann cells in the nerve conduit group (CO), Schwann cells only in the nerve conduit group (SC), neural stem cells only in the nerve conduit group (NSC-only), nerve conduit group (null), autologous nerve graft group (autograft) and sham operation group (sham). Regenerated nerves were evaluated by histological and functional assessment at 8 and 12 weeks after surgery. The diameter and area of the regenerated myelin sheath, as well as the secretion of brain-derived neurotrophic factor and glial cell-derived neurotrophic factor in laryngeal muscle or regenerated nerve tissue in the CO group, were significantly better than they were in the SC, NSC-only and null groups (all P values < 0.05). Immunofluorescence showed that the CO group had significantly more neurofilament-200 immunoreactive and S-100 immunoreactive fibres than the SC, NSC-only and null groups (all P values < 0.05). The performance of the CO groups and autograft groups was found to be similar by laryngoscopy. Arytenoid cartilage motion recovery in these two groups was significantly better than it was in the other groups (all P values < 0.05). Our results indicated that co-culture of Schwann cells and NSCs in laminin-chitosan-PLGA conduits might promote injured nerve regeneration. This method might be a promising alternative for defective nerve repair.





Similar content being viewed by others
References
Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8(4):243–52.
Xie J, Jin B, Li DW, Shen B, Gong N, Zhang TZ, et al. Effect of laminin-binding BDNF on induction of recurrent laryngeal nerve regeneration by miR-222 activation of mTOR signal pathway. Am J Transl Res. 2015;7:1071–80.
Hayward NJ, Grodski S, Yeung M, Johnson WR, Serpell J. Recurrent laryngeal nerve injury in thyroid surgery: a review. ANZ J Surg. 2013;83(1-2):15–21.
Choi J-S, Oh SH, An H-Y, Kim Y-M, Lee JH, Lim J-Y. Functional regeneration of recurrent laryngeal nerve injury during thyroid surgery using an asymmetrically porous nerve guide conduit in an animal model. Thyroid. 2014;24:52–9.
Dos Santos FP, Peruch T, Katami SJV, Martini APR, Crestani TA, Quintiliano K, et al. Poly (lactide-co-glycolide) (PLGA) scaffold induces short-term nerve regeneration and functional recovery following sciatic nerve transection in rats. Neuroscience. 2019;396:94–107.
Safa B, Buncke G. Autograft substitutes: conduits and processed nerve allografts. Hand Clin. 2016;32:127–40.
Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77–85.
Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin.2013;29(3):331–48.
Li Y, Yu Z, Men Y, Chen X, Wang B. Laminin-chitosan-PLGA conduit co-transplanted with Schwann and neural stem cells to repair the injured recurrent laryngeal nerve. Exp Ther Med. 2018;16:1250–8.
Yu Z, Men Y, Dong P. Schwann cells promote the capability of neural stem cells to differentiate into neurons and secret neurotrophic factors. Exp Ther Med. 2017;13:2029–35.
Huang YC, Huang CC, Huang YY, Chen KS. Surface modification and characterization of chitosan or PLGA membrane with laminin by chemical and oxygen plasma treatment for neural regeneration. J Biomed Mater Res A. 2007;82:842–51.
Zhao J, Chen LJ, Yu K, Chen C, Dai YL, Qiao XY, et al. Effects of chitosan coating on biocompatibility of Mg-6%Zn-10%Ca3(PO4)2 implant. Trans Nonferrous Met Soc China. 2015;25:824–31.
Kim I-Y, Seo S-J, Moon H-S, Yoo M-K, Park I-Y, Kim B-C, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv.2008;26(1):1–21.
Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–49.
Gilchrist SE, Lange D, Letchford K, Bach H, Fazli L, Burt HM. Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J Control Release. 2013;170:64–73.
Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17(3):247–89.
Carriel V, Garzón I, Alaminos M, Cornelissen M. Histological assessment in peripheral nerve tissue engineering. Neural Regen Res. 2014;9:1657–60.
Yongzhi M, Yusheng C, Yu L, Pin D, Ziwei Y. Cytocompatibility research of schwann cells and neural stem cells co-seeded on electron spinning chitosan-PLGA. J Shandong Univ. 2015;29:16–9.
Cao Q-L, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177:349–59.
An Y-H, Wan H, Zhang Z-S, Wang H-Y, Gao Z-X, Sun M-Z, et al. Effect of rat Schwann cell secretion on proliferation and differentiation of human neural stem cells. Biomed Environ Sci. 2003;16:90–4.
Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24.
Anisimov SV. [Cell therapy for Parkinson’s disease: IV. Risks and future trends]. Adv Gerontol. 2009;22:418–39.
Stadnikov AA, Shevliuk NN. [Stem cells and reparative regeneration in mammalian postnatal ontogenesis]. Morfologiia. 2006;130:84–8.
Acknowledgements
The authors would like to thank Donghua University, College of Textiles, for their technical support.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81800893 and 81170925).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Y., Men, Y., Wang, B. et al. Co-transplantation of Schwann cells and neural stem cells in the laminin-chitosan-PLGA nerve conduit to repair the injured recurrent laryngeal nerve in SD rats. J Mater Sci: Mater Med 31, 99 (2020). https://doi.org/10.1007/s10856-020-06436-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06436-z