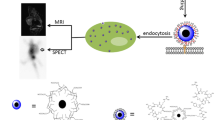
Abstract
Development of biocompatible and multifunctional nanoprobes for tumor targeting, imaging, and therapy still remains a great challenge. Herein, gold nanoparticles (AuNPs) were synthesized in the cavity of horse spleen apoferritin protein (HoSAF) and protein surface was labeled with 2-amino-2-deoxy-glucose (2DG) as a cell surface glucose transport protein specific targeting probe to study the feasibility of its usage as a computer tomography (CT) contrast agent with tumor targeting capability through in vitro experiments. 2DG conjugated and gold-loaded apoferritin (Au-HoSAF-2DG) nanoparticles (NPs) showed selective targeting for human breast adenocarcinoma (MCF-7) cells when compared to normal breast (MCF-10A) cells. This AuNP-based imaging agent was found to be non-cytotoxic in a given concentration range with an apoptotic effect upon longer exposure times towards MCF-7 cells, while MCF-10A cells were affected less. This selective cell death would also be useful for further cancer treatments with the ability of X-ray attenuation in in vitro X-ray and computed tomography (CT) imaging.
Graphic abstract






Similar content being viewed by others
References
Curry T, Kopelman R, Shilo M, Popovtzer R (2014) Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol Imaging 9:53–61
Kim D, Jeong YY, Jon S (2010) A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 4(7):3689–3696
Huang Y, Liu Q, Wang Y, He N, Zhao R et al (2019) Gold nanorods functionalized by a glutathione response near-infrared fluorescent probe as a promising nanoplatform for fluorescence imaging guided precision therapy. Nanoscale 11(25):12220–12229
Zhang W, Wang Y, Sun X, Wang W, Chen L (2014) Mesoporous titania based yolk-shell nanoparticles as multifunctional theranostic platforms for SERS imaging and chemo-photothermal treatment. Nanoscale 6(23):14514–14522
Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J et al (2008) Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett 8(12):4593–4596
Huang P, Bao L, Zhang C, Lin J, Luo T et al (2011) Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials 32:9796–9809
Her S, Jaffray DA, Allen C (2017) Gold nanoparticles for application in cancer radiotherapy: mechanism and recent advancements. Adv Drug Deliv Rev 109:84–101
Melo AFAA, Hassan A, Macedo LJA, Osica I, Shrestha LK et al (2019) Microwires of Au-Ag nanocages patterned via magnetic nanoadhesives for investigating proteins using surface enhanced infrared absorption spectroscopy. Appl Mater Interfaces 11(20):18053–18061
Wang Y, Gao Z, Liu B, Xia X (2019) Peroxidase-AgAu hybrid nanocages as signal transducers for sensitive plasmonic colorimetric sensing. J Mater Chem C 7(48):15179–15187
Gao M, Yu F, Lv C, Choo J, Chen L (2017) Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem Soc Rev 46(8):2237–2271
Wang R, Deng J, He D, Yang E (2019) PEGylated hollow gold nanoparticles for combined X-ray radiation and photothermal therapy in vitro and enhanced CT imaging in vivo. Nanomed Nanotechnol Biol Med 16:195–205
Komeri R, Maya S, Unnikrishnan BS, Sreekutty J, Preethi GU et al (2019) Galactoxyloglucan-modified gold nanocarrier of doxorubicin for treating drug-resistant brain tumors. Appl Nano Mater 2(10):6287–6299
Hainfeld JF, O’Connor MJ, Dilmanian FA, Slatkin DN, Adams DJ et al (2011) Micro-CT enables microlocalisation and quantification of Her2-targeted gold nanoparticles within tumour regions. Br J Radiol 84(1002):526–533
Chanda N, Kattumuri V, Shukla R, Zambrea A, Katti K et al (2010) Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. PNAS 107(19):8760–8765
Zhang L, Swift J, Butts CA, Yerubandi V, Dmochowski IJ (2007) Structure and activity of HoSAF-stablized gold nanoparticles. J Inorg Biochem 101:1719–1729
Fan R, Chew SW, Cheong VV, Orner BP (2010a) Fabrication of gold nanoparticles inside unmodified horse spleen HoSAF. Small 6(14):1483–1487
Sennuga A, Marwijk J, Whiteley CG (2013) Multiple fold increase in activity of ferroxidase–HoSAF complex by silver and gold nanoparticles. Nanomed Nanotechnol Biol Med 9:185–193
Petrucci OD, Buck DC, Farrer JK, Watt RK (2014) A ferritin mediated photochemical method to synthesize biocompatible catalytically active gold nanoparticles: size control synthesis for small (~2 nm), medium (~7 nm) or large (~17 nm) nanoparticles. RSC Adv 4:3472–3481
Kim M, Rho Y, Jin KS, Ahn B, Jung S (2011) ph-Dependent structures of ferritin and HoSAF in solution: disassembly and reassembly. Biomacromol 12:1629–1640
Ali MRK, Panikkanvalappil SR, El-Sayed MA (2014) Enhancing the efficiency of gold nanoparticles treatment of cancer by increasing their rate of endocytosis and cell accumulation using rifampicin. J Am Chem Soc 136:4464–4467
Aslan TN, Aşık E, Volkan M (2016) Preparation and labeling of surface-modified magnetoferritin protein cages with a rhenium (I) carbonyl complex for magnetically targeted radiotherapy. RSC Adv 6:8860–8869
Vannucci L, Falvo E, Fornara M, Micco PD, Benada O et al (2012) Selective targeting of melanoma by PEG-masked protein-based multifunctional nanoparticles. Int J Nanomed 7:1489–1509
Hea J, Fana K, Yan X (2019) Ferritin drug carrier (FDC) for tumor targeting therapy. J Control Release 311–312:288–300
Xu C, Tung GA, Sun S (2008) Size and concentration effect of gold nanoparticles on X-ray attenuation as measured on computed tomography. Chem Mater 20:4167–4169
Wang Z, Wu L, Cai W (2010) Size-tunable synthesis of monodisperse water-soluble gold nanoparticles with high X-ray attenuation. Chem-Eur J 16:1459–1463
Xi D, Dong S, Meng X, Lu Q, Meng L et al (2012) Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv 2:12515–12524
Chen H, Zhang S, Xu C, Zhao G (2016) Engineering protein interfaces yields ferritin disassembly and reassembly under benign experimental conditions. Chem Comm 00:1–3
Monti DM, Ferraro G, Petruk G, Maiore L, Pane F et al (2017) Ferritin nanocages loaded with gold ions induce oxidative stress and apoptosis in MCF-7 human breast cancer cells. Dalton Trans 46:15354–15362
Sun C, Yang H, Yuan Y, Tian X, Wang L et al (2011) Controlling assembly of paired gold clusters within HoSAF nanoreactor for in vivo kidney targeting and biomedical imaging. J Am Chem Soc 133:8617–8624
Kramer RM, Li C, Carter DC, Stone MO, Naik RR (2004) Engineered protein cages for nanomaterial synthesis. J Am Chem Soc 126:13282–13286
Aydoğan B, Li J, Rajh T, Chaudhary A, Chmura SJ (2010) AuNP-DG: Deoxyglucose labeled gold nanoparticles as X-ray computed tomography contrast agents for cancer imaging. Mol Imag Biol 12:463–467
Shan XH, Wang P, Xiong F, Gu N, Hu H (2015) MRI of high-glucose metabolism tumors: a study in cells and mice with 2-DG-modified superparamagnetic iron oxide nanoparticles. Mol Imag Biol 18(1):24–33
Xiong F, Zhu Z, Xiong C, Hua X, Shan X et al (2012) Preparation, characterization of 2-deoxy-D-glucose functionalized dimercaptosuccinic acid-coated maghemite nanoparticles for targeting tumor cells. Pharm Res 29(4):1087–1097
Shan XH, Hu H, Xiong F, Gu N, Geng XD et al (2012) Targeting Glut1-overexpressing MDA-MB-231 cells with 2-deoxy-D-glucose modified SPIOs. Eur J Radiol 81:95–99
Rajendran JG, Mankoff DA, O’Sullivan F, Peterson LM, Schwartz DL et al (2004) Hypoxia and glucose metabolism in malignant tumors: Evaluation by [18F] Fluoromisonidazole and [18F] Fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 10(7):2245–2252
Luciani A, Olivier JC, Clement O, Siauve N, Brillet PY et al (2004) Glucose-receptor MR imaging of tumors: study in mice with PEGylated paramagnetic niosomes. Radiology 231(1):135–142
Aşık E, Aslan TN, Volkan M, Güray NT (2016) 2-Amino-2-deoxy-glucose conjugated cobalt ferrite magneticnanoparticle (2DG-MNP) as a targeting agent for breast cancer cells. Environ Toxicol Pharmacol 41:272–278
Zheng B, Xue M, Zhang X, Tian N, Wang D (2020) Breast cancer diagnosed by MRI using Mesoporous TiO2-coated (Fe3O4) nanoparticles. J Nanosci Nanotechnol 20:6561–6567
Wonga KKW, Colfenb H, Whiltona NT, Douglas T, Mann S (1999) Synthesis and characterization of hydrophobic ferritin proteins. J Inorg Biochem 76:187–195
Anthon GE, Barrett DM (2002) Determination of Reducing Sugars with 3-Methyl-2-benzothiazolinonehydrazone. Anal Biochem 305:287–289
Hennequin B, Turyanska L, Ben T, Beltrán AM, Molina SI, Li M et al (2008) Aqueous near-infrared fluorescent composites based on apoferritin-encapsulated PbS quantum dots. Adv Mater 20:3592–3596
Shin Y, Dohnalkova A, Lin Y (2010) Preparation of homogeneous gold-silver alloy nanoparticles using the apoferritin cavity as a nanoreactor. J Phys Chem C 114:5985–5989
Fan R, Chew SW, Cheong VV, Orner BP (2010b) Fabrication of gold nanoparticles inside unmodified horse spleen apoferritin. Small 6(14):1483–1487
Chen H, Zhang S, Xub C, Zhao G (2016) Engineering protein interfaces yields ferritin disassembly and reassembly under benign experimental conditions. Chem Commun 52:7402–7405
Swift J, Butts CA, Cheung-Lau J, Yerubandi V, Dmochowski IJ (2009) Efficient self-assembly of archaeoglobus fulgidus ferritin around metallic cores. Langmuir 25(9):5219–5225
Sennuga A, van Marwijk J, Whiteley CG (2013) Multiple fold increase in activity of ferroxidase-HoSAF complex by silver and gold nanoparticles. Nanomedicine 9:185–193
Vashist SK (2012) Comparison of 1-Ethyl-3-(3-Dimethylaminopropyl) carbodiimide based strategies to crosslink antibodies on amine-functionalized platforms for immunodiagnostic applications. Diagnostics 2:23–33
Pacławski K, Streszewski B, Jaworski W, Luty-Błocho M, Fitzner K (2012) Gold nanoparticles formation via gold(III) chloride complex ions reduction with glucose in the batch and in the flow microreactor systems. Colloids Surf A Physicochem Eng Aspects 413:208–215
Kwon KC, Ryu JH, Lee JH, Lee EJ, Kwon IC et al (2014) Proteinticle/gold core/shell nanoparticles for targeted cancer therapy without nanotoxicity. Adv Mater 26:6436
Li X, Qiu L, Zhu P, Tao X, Imanaka T et al (2012) Epidermal growth factor-ferritin H-chain protein nanoparticles for tumor active targeting. Small 8:2505
Lianga M, Fana K, Zhou M, Duana D, Zhenga J (2014) H-ferritin–nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. PNAS 111(41):14900–14905
Kitagawa T, Kosuge H, Uchida M, Iida Y, Dalman RL et al (2017) RGD targeting of human ferritin iron oxide nanoparticles enhances in vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm. J Magn Reson Imaging 45:1144–1153
Lee NK, Lee FJ, Kim S, Nama G, Kiha M et al (2017) Ferritin nanocage with intrinsically disordered proteins and affibody: a platform for tumor targeting with extended pharmacokinetics. J Control Release 267:172–180
Jennifer R, Charlton JR, Pearl VM, Denotti AR, Lee J et al (2016) Biocompatibility of ferritin-based nanoparticles as targeted MRI contrast agents. Nanomed Nanotechnol Biol Med 12:1735–1745
Kwon KC, Ko KY, Lee J, Lee EJ, Kim K, Lee J (2016) Enhanced in vivo tumor detection by active tumor cell targeting using multiple tumor receptor-binding peptides presented on genetically engineered human ferritin nanoparticles. Small 12:4241–4253
Wong RSY (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87
Acknowledgements
We would like to thank Arda Büyüksungur for CT imaging and Seçkin Öztürk for HRTEM imaging at METU.
Funding
This study was funded by Necmettin Erbakan University Research Funding Center (BAP-Project No: 191215003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
775_2020_1830_MOESM1_ESM.pdf
Suppl. Fig. 1 SEC absorbance profiles of HoSAF a) before AuNP formation, b) after AuNP formation with 4000 Au/cage. Elution was monitored both at 280 nm (protein) and 525 nm (AuNP). Suppl. Fig. 2 SEC absorbance profile of Au-HoSAF-2DG. Elution was monitored at both 280 nm (protein) and 525 nm (AuNP). Suppl. Fig. 3 Cell viability of MCF-10A (left) and MCF-7 (right) cells after treatment with 2 different concentrations (0.5 (empty column) and 1.0 mg/mL (filled column)) of HoSAF, Au-HoSAF and Au-HoSAF-2DG NPs for 24 h, 48 h and 72 h (top to bottom). Suppl. Fig. 4 The flow cytometry analysis results of control and after Au-HoSAF-2DG NP treatment at concentration of 1.0 mg/mL for 24 h, 48 h and 72 h of a) MCF-7 and b) MCF-10 A cells. Area A1 and A2 (left, bottom and up) reveals viable and dead cell (necrotic), A3 and A4 (right, top and bottom) correspond to cells undergoing early and late apoptosis, respectively Suppl. Fig. 1 SEC absorbance profiles of HoSAF a) before AuNP formation, b) after AuNP formation with 4000 Au/cage. Elution was monitored both at 280 nm (protein) and 525 nm (AuNP). Suppl. Fig. 2 SEC absorbance profile of Au-HoSAF-2DG. Elution was monitored at both 280 nm (protein) and 525 nm (AuNP). Suppl. Fig. 3 Cell viability of MCF-10A (left) and MCF-7 (right) cells after treatment with 2 different concentrations (0.5 (empty column) and 1.0 mg/mL (filled column)) of HoSAF, Au-HoSAF and Au-HoSAF-2DG NPs for 24 h, 48 h and 72 h (top to bottom). Suppl. Fig. 4 The flow cytometry analysis results of control and after Au-HoSAF-2DG NP treatment at concentration of 1.0 mg/mL for 24 h, 48 h and 72 h of a) MCF-7 and b) MCF-10 A cells. Area A1 and A2 (left, bottom and up) reveals viable and dead cell (necrotic), A3 and A4 (right, top and bottom) correspond to cells undergoing early and late apoptosis, respectively (PDF 686 kb)
Rights and permissions
About this article
Cite this article
Aslan, T.N., Aşık, E., Güray, N.T. et al. The potential application of gold-apoferritin nanocages conjugated with 2-amino-2-deoxy-glucose for imaging of breast cancer cells. J Biol Inorg Chem 25, 1139–1152 (2020). https://doi.org/10.1007/s00775-020-01830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-020-01830-y