Abstract
Background
Groundwater is typically over-saturated in CO2 with respect to atmospheric equilibrium. Irrigation with groundwater is a common agricultural practice in many countries, but little is known about the fate of dissolved inorganic carbon (DIC) in irrigation groundwater and its contribution to the CO2 emission inventory from land to the atmosphere. We performed a mesocosm experiment to study the fate of DIC entering agricultural drainage channels in the North China Plain. Specifically, we aimed to unravel the effect of flow velocity and nutrient on CO2 emissions.
Results
All treatments were emitting CO2. Approximately half of the DIC in the water was consumed by TOC production (1–16%), emitted to the atmosphere (14–20%), or precipitated as calcite (CaCO3) (14–20%). We found that DIC depletion was stimulated by nutrient addition, whereas more CO2 evasion occurred in the treatments without nutrients addition. On the other hand, about 50% of CO2 was emitted within the first 50 h under high flow velocity. Thus, in the short term, high nutrient levels may counteract CO2 emissions from drainage channels, whereas the final fate of the produced biomass (burial versus mineralization to CO2 or even CH4) determines the duration of the effect.
Conclusion
Our study reveals that both hydrology and biological processes affect CO2 emissions from groundwater irrigation channels. The estimated CO2 emission from total groundwater depletion in the North China Plain is up to 0.52 ± 0.07 Mt CO2 year−1. Thus, CO2 emissions from groundwater irrigation should be considered in regional CO2 budgets, especially given that groundwater depletion is expected to acceleration in the future.
Similar content being viewed by others
Background
Groundwater is a critical water resource around the globe ensuring food and water security [1]. Irrigation with groundwater for agricultural activities is a common practice in many arid and semi-arid regions [2]. However, over exploitation of groundwater has led to severe groundwater depletion in several regions of the world [3]. Areas most affected by groundwater depletion are California and Midwest in the US, Northern India, and the North China Plain (NCP) [4]. In the NCP approximately 70% of the irrigated area is currently groundwater-fed [5, 6] which causes the groundwater level to drop by more than 2 cm per year [7]. While several studies focus on the importance and influence of the dramatic groundwater level drop, the further fate of the pumped irrigation water is much less studied [8].
Groundwater is typically 10- to 100-fold over-saturated with CO2 [9], even up to 250 times the atmospheric equilibrium value [10]. Thus, if it is pumped to the surface, CO2 is released. First estimates show that CO2 emissions from groundwater pumping probably represent a globally significant source of CO2 [11], with both CO2 liberation from the water and CO2 production due to pump energy generation contributing to the negative climate impact of groundwater irrigation [12]. CO2 has been recognized as the dominant driver of climate change resulting in tremendous deteriorative impacts on the environment and society at a global scale [13]. As one of the major emission sources of CO2, agricultural land plays an important role in the global carbon cycle [14]. Direct CO2 emissions from agriculture have been well-documented in many regions [15,16,17]. However, indirect emission sources, such as irrigation drainages and rivers receiving drainage water, may account for a large part of the uncertainties in the carbon budgets of agricultural ecosystems [18,19,20,21].
If CO2 containing water equilibrates with the atmosphere, not only the dissolved CO2 but also other species of the carbonate system have to be considered [22]. Existing estimates of CO2 emissions from groundwater pumping assume complete evaporation of the pumped water, leaving solutes and solids at the surface. Under such conditions, each molecule of CO2 outgassing is theoretically produced from two molecule of carbonate hardness (refers to HCO3− + CO32−) to re-equilibrate with atmospheric CO2 accompanied by one molecule of carbonate precipitation simultaneously [11, 23]. Thus, maximum CO2 liberation from groundwater dissolved inorganic carbon could be calculated from the bicarbonate content of the groundwater [9]. When a large part of dissolved inorganic carbon (DIC) containing irrigation water drains into surface waters, DIC may have four different fates: evasion as CO2, carbonate precipitation, fixation into biomass by TOC production, and downstream transport [24].
Streams are recognized as important CO2 sources to the atmosphere [25, 26]. In streams the distribution among the four fates of groundwater inorganic carbon is controlled by both hydrological and biochemical processes and their complex interactions [10, 27]. Flow velocity, characterized by fluctuating hydraulic conditions due to flood irrigation or excessive utilization of water resources, usually results in enhanced carbon export from terrestrial and wetland habitats to fluvial networks that subsequently become a source of CO2 [25, 28, 29]. Turbulence at the air–water interface, which is dependent on the river geomorphology and the river flow, affects the gas transfer coefficient of CO2, which ultimately determines fluvial CO2 emissions [30,31,32]. On the other hand, increasing nutrient levels stimulate biological processes such as algal growth, which decrease DIC concentration by converting CO2 to organic carbon via photosynthesis [33]. Agricultural drainage water is typically high in inorganic nutrients. Thus, our hypothesis is that both nutrients and flow velocity mediate the relative importance of uptake versus outgassing of CO2 accompanied with calcite precipitation from the dense agricultural drainage channels transporting pumping groundwater.
However, quantifying these processes of DIC depletion remains challenging. It is difficult to disentangle the effects of both factors on the fate of inorganic carbon from field observations only, primarily due to uncontrolled environmental conditions. We, therefore, performed a mesocosm experiment in the field to explore how CO2 emissions from irrigation groundwater respond to nutrients and flow velocity treatments. The goals of this study are to (1) quantitatively testify the effects of nutrients and flow velocity on CO2 emissions and the carbon budget of irrigation drainage channels in a typical agricultural region in China, and (2) provide implications to CO2 emission potential from the irrigation groundwater in the NCP as well as other similar regions worldwide.
Methods
Study area
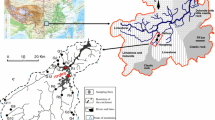
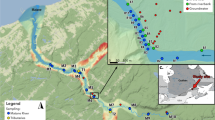
Our study was conducted in the Yucheng Comprehensive Experiment Station of Dezhou Irrigation District (DID) between 115°45′–117°36′ E and 36°24′–38°00′ N, located in the North China Plain. The annual-averaged precipitation is 587 mm (ranges from 286 to 1034 mm). Precipitation occurs mostly from June to September, which accounts for 75% of the total annual amount. Annual evaporation is between 900 and 1400 mm and annual mean temperature is 12.8 °C [34]. The groundwater for agricultural irrigation is characterized by high alkalinity and enriched with agricultural nutrients when it enters the drainage channels [35]. Groundwater bicarbonate concentrations in the NCP can range between 1.55 and 7.65 mmol L−1 [36]. The ditches and canals are mainly artificial and lined with concrete.
Historically, DID has been characterized by anthropogenic activities, particularly intensive agriculture development, which has been causing a high demand of water resources in the NCP [37]. Irrigation drainages were built during the twentieth century to increase crop yield in the salinized soil by diverting water from the lower reaches of the Yellow River to DID. Given that the surface water cannot fulfill the demand from agricultural and industrial production, over-exploitation of groundwater had been frequently observed through the sharp decrease of the groundwater table and heavy pollution therein [38]. To eliminate the influence of soil salinity on crop growth, flooding irrigation is still commonly applied among local farmers. This enhances nutrient inputs from irrigated groundwater into the surface water network that subsequently become a source of CO2. Based on farmer’s practice when they irrigate the crops, our experiment simulate that stream drainage collected from groundwater-irrigated agricultural land. For better representation of local groundwater, we used groundwater from a local irrigation well as inlet water to our experimental system.
Mesocosm setup
Cubic mesocosms (volume 0.2 m3) were constructed to mimic drainage channels. The physical design of our mesocosms follows that from Petersen et al. [39]. The systems are composed of sixteen cuboid polyvinyl chloride plastic tanks with 0.9 m in length, 0.3 m in width, and 0.6 m in depth. Depth of water was set to 0.5 m. Mesocosms were placed outdoor and remained open and in contact with the field atmosphere to ensure natural light and weather conditions. The containers were sunk into the ground to buffer heating from ambient atmosphere temperature and solar radiation. The electrical wiring for power supplying was also buried. The upper edge of the tanks was 10 cm above the ground so that no surface runoff could flow into containers during rainstorms. The containers allow a factorial design combining two nutrient levels with two flow velocity levels in four replicates (Additional file 1: Figure S1). The tanks were equipped with two alternative submerged pumps to mimic river flowing. Each mesocosm had an individual pump (65 W or 15 W) to provide continuous flowing condition and to preclude water pressure differences of connected pipes when sharing the same pump under the same treatment. The pumps were placed in mesh cages to prevent snails, insects, and floating fragments from suction into the pump and to reduce the risk of clogging. The inlet of the pump was on the shorter side of the tank to simulate a unit of water channel (Fig. 1). We set the length to width ratio as 3:1 to establish a realistic flow regime in the central part of our setting. The middle section along the longitudinal direction of these tanks can be considered a homogeneous flow field with simple physical boundaries.
Experimental procedure
Groundwater was collected from a nearby agricultural irrigation well from a depth of 5 m and pumped into the pre-cleaned tanks without sediments. Nutrients were added into the tanks at the beginning of the experiment for the high-nutrient treatment, while low-nutrient tanks received no additional nutrients. Particularly, high-nutrient tanks were instantaneously fed with 40 mg P m−3 and 4000 mg N m−3 as K2HPO4 and Ca(NO3)2, respectively. We set the nutrient enrichment level by mimicking the two alternative states existing in the local drainage system. The nitrate and ammonia concentrations of the study groundwater well were approximately 0.02 mg N L−1 and 5.8 μg N L−1, respectively, while the nitrate concentration of drainages in a field survey in Yucheng (on 16th and 17th of September) varied from 0.02 to 0.42 mg N L−1 with a mean value of 0.17 mg N L−1 (Table 1). Other parameters in the experimental water were comparable to that in the surface water as well (Table 1). We designed the initial nitrate concentration for the high nutrient experimental group at a level above the average nitrate concentration (0.3 mg N L−1). Phosphorous was added with the N:P molar ratio of 10:1 following Lone et al. [40].
We choose flow velocities in surrounding drainage channels as a reference. Flow velocity of local drainages varied from 0.001 to 0.340 m s−1 during our field sampling. Accordingly, two levels of flow velocity were set to 0.1 and 0.4 m s−1. Pumps were cleaned manually every 3 days. It is expected that there was little CO2 degassing through pumping itself, since the submerged pumps were tested for air-tightness before usage and it is expected that any artifact would be equal between low and high nutrient treatments, because the same pumps were used.
Overall, there were four (2 × 2) treatments with 4 replicates each in our experimental design, which are denoted as: high nutrient and high flow velocity (A + H); high nutrient and low flow velocity (A + L); low nutrient and high flow velocity (N + H); and low nutrient and low flow velocity (N + L).
The experiments was conducted between 31st August and 15th September 2018. Sampling and measurements of the physical and chemical parameters was performed at 0, 4, 16, 32, 64, 112, 160, 280 h. The experiment measurement was finished after 12 days when the CO2 concentration had reached equilibrium with the atmosphere. Due to the mesocosm malfunction, the N + H treatment has only one replicate after 50 h.
Sampling and analysis
Water temperature, dissolved oxygen (DO), and electrical conductivity (EC) were measured using multi-parameter probes (Hach H40d, USA). Water level and flow velocity were recorded using a flow meter (HR-2, China) in parallel to each sampling. Water samples were collected to quantify concentrations of nitrate, ammonium, DIC, alkalinity, and TOC. For determination of NO3−–N and NH4+–N concentration, water samples were filtered using 0.45 μm filters by spectrophotometry (TU-1810DSPC, China). Calibration curves were produced using reference samples according to quality control standards and were then applied to evaluate data from each set of samples. Reagents, procedural blanks, and samples were measured twice in parallel, with average values reported. The differences between two replicates were within 5% of the mean value for all samples. NO3− and NH4+ concentrations could be measured at precisions of 2.6% and 8.6%, respectively. DIC and TOC were analyzed with a Vario TOC Analyzer (Elementar Analysensysteme GmbH, Germany). The detection limits of DIC and TOC were 4 and 1 μg L−1 with precisions of 1.5% and 5%, respectively. Alkalinity was determined by titration with ~ 0.01 M H2SO4 at a precision of 6%. While the measured TOC data could not be directly used. We used two methods to quantify CO2 emissions during our experiments: a carbon budget approach and direct flux measurements using floating chambers (see Additional file 1 for method details).
Calculation of C budgets
Changes of DIC in the mesocosms can be separated into three parts: carbon dioxide evasion, calcite precipitation, and total organic carbon (TOC) production (Fig. 1):
To verify whether carbonate precipitation was likely to occur within the mesocosm, we calculated calcite saturation indices (SIs) using PHREEQC [41]. Because we did not measure calcium concentrations in the mesocosm, we used historical rainy season data from irrigation ditches in Dezhou District [42] and derived possible SIs with 1.28 ± 0.16 (mean value ± standard deviation). Therefore, we assumed calcite precipitation occurred in all mesocosms, with 1 mol each of CO2 outgassed and CaCO3 precipitated from 2 mol of HCO3− according to Wood and Hyndman [11]:
CO2 evasion depends on CO2 concentration and the physical gas transfer velocity [32]. The CO2 concentration is affected by the thermodynamic carbonate equilibrium. TOC formation is a biological process that converts inorganic carbon into organic matter. At any time, the variation for DIC is denoted as:
Equation (3) is based on our hypothesis that CO2 in the system was over-saturated and TOC was generated during the experiment, \({\text{DIC}}_{0}\) and \({\text{TOC}}_{0}\) is the amount of DIC and TOC at the initial time, respectively, and \({\text{DIC}}_{t}\) and \({\text{TOC}}_{t}\) is the amount of DIC and TOC at time t, respectively. \(\Delta {\text{CO}}_{2}\) and \(\Delta {\text{CaCO}}_{3}\) is the amount of CO2 outgassing and calcite precipitating during time 0 to time t, respectively. Combined with Eq. (2),
This is a conservative estimate as we note that the ratio of \(\Delta {\text{CO}}_{2} :\Delta {\text{CaCO}}_{3}\) can range from 1.18 to 1.88 [9]. The CO2 evasion could be deduced by the difference between the variation amount of DIC and the variation amount of TOC, re-arranging Eqs. (3) with (4) gives:
Because TOC data were not available, we estimated the TOC generation indirectly from total inorganic nitrogen (TIN) consumption. Assuming the molar C:N ratio in freshwater being higher than the Redfield ratio of 106:16 [43, 44], TOC production could be estimated from the consumption of nitrate and ammonium, which are the main species of nitrogen in the groundwater system with nitrate accounting for the majority, based on a C:N ratio of 10:1 [45]. We assume that denitrification as a process that removes N from the system can be neglected in our case, because our system was turbulent and well aerated.
Our mass balance was calculated by multiplying the concentrations with the varying water volume in the setup (mesocosm bottom area × decreasing water level with negligible volume of the water in pipes and pump), so the evaporation would not influence our result.
We performed a series of analysis of variance (ANOVA) to explore the patterns of DIC, TOC, and CO2 and their relationship to nutrient and flow velocity. The concentration of CO2 at given DIC and alkalinity was calculated using standard dissociation constants of the carbonate system [46]. Data were log-transformed to satisfy assumptions of residuals normal distribution. ANOVA was applied to test for overall statistical differences among treatments. The ratio to the initial amount of the above parameters for each of the mesocosm was carried out to test for the treatment effects using ANOVA. In the model, nutrient and flow velocity were treated as the fixed effect, and temporal pseudo-replication from repeated sampling over time was considered as nested within each mesocosm as random effects. The comparison of DIC and CO2 changes is given by the different slope of a first-order reaction of log10 DIC or log10 CO2 over time to test for statistical differences for each treatment and mesocosm. All calculations, statistical analysis, and data visualization were performed using R version 3.5.1 [47].
Results
Significant differences among four treatments
The differences of flow velocities and nutrients among the four treatments were significant at the beginning of the experiment (Table 2). The nitrate concentration was similar between high and low flow with a larger variation range in high flow treatments. Because of some clogging of the pumps, the flow velocity fluctuated at a larger range at high flow compared to the low flow velocity.
At first, DO saturation increased exponentially over time in all mesocosms (Fig. 2). The initial DO concentration at high flow was higher than that at low flow at the starting time, probably because more turbulences at high flow mixed more oxygen into the water before the first sampling. DO saturation varied dissimilarly over time and treatments as well. Mesocosms with nutrient additions developed a clear over-saturation, showing that TOC production was stimulated by nutrient additions. The A + L treatment reached the highest over-saturation. On the contrary, the control mesocosms rapidly reached equilibrium with atmosphere. As expected, equilibration was faster at high flow.
EC in the high flow velocity treatments showed a similar trend with a sharp decrease from 2.24 to 1.95 mS cm−1 in the first 150 h after which it stayed constant (Fig. 2). The low flow velocity treatments showed a steadily decrease from 2.24 to 1.95 mS cm−1 from the start to the end of experiment.
DIC and alkalinity were used to calculate the CO2 concentrations at the beginning and in the end. At the end of the experiment in the high nutrient treatment, pCO2 in water was below that in the atmosphere, indicating CO2 uptake. Assuming alkalinity has a linear decrease over time accompanied with known DIC changes (Fig. 3a–d), we calculated the pCO2 at any time in each mesocosm using the CO2SYS program [48]. Thus, the time when pCO2 in each mesocosm was equal to pCO2 in the atmosphere (pCO2air = 395 μatm) could be estimated. After about 125 and 235 h, the systems switched to CO2 uptake under the A + H and A + L treatments, respectively (Additional file 1: Figure S2). The N + L treatment reached CO2 equilibration with atmosphere until the end of experiment.
DIC and TOC
The DIC amount in all treatments decreased continuously by 31–48% during the experimental period (Fig. 3). Decrease of DIC was largest in the A + H treatment and lowest in the N + L treatment. The amount of DIC in the system was significantly different between the two nutrient treatments (p = 0.041), as well as between the two flow velocities (p = 0.020). DIC in the N + H treatment decreased rapidly at the beginning of the experiment (particularly at the first 50 h). The decreasing rate of DIC under the A + H treatment surpassed that under the N + H treatment, while the rate was relatively slow but steadily after 170 h. The rate of DIC decrease was also strongly related to flow velocity (p = 0.006) but had insignificant relationship with nutrients. Moreover, there was a significant interaction of flow velocity and nutrients in the DIC decrease slope showing a combined effect between the two factors (p = 0.043).
The TOC increase in the high nutrient treatments was evidently much higher than the one in the treatment without nutrients addition (Fig. 3) and independent of flow velocity. In the low nutrient treatments, TOC did not significantly change during the experiment, indicating little TOC production in these systems. This is consistent with the DO data which showed no O2 oversaturation in the low nutrient treatments (Fig. 2).
CO 2 evasions among four treatments
All mesocosms were net sources of CO2 during the experimental period (Fig. 4). Always positive fluxes (except at the end of the experiment) were confirmed by our floating chamber measurements (Fig. 4e–h). The high flow treatments reached CO2 equilibrium between water and atmosphere faster (Additional file 1: Figure S2). The rate of CO2 change also shows the significant difference between flow velocity treatments (p = 0.024). High flow velocity sped up CO2 evasion. More CO2 was emitted significantly under the high flow velocity (p = 0.005) and low nutrient condition (p = 0.096). CO2 emission from the low nutrient treatments was 35% higher than that in the high nutrient treatments in average.
Cumulative CO2 emissions in each container over time among four treatments calculated from DIC (a–d) and from floating chamber measurements (e–h), the dashed lines show the exponential fits (p < 0.001) and colored areas indicate 95% confidence level, the red dots indicate the negative CO2 fluxes, namely there was CO2 uptake occurring
CO2 evasions of the N + H treatment increased rapidly during the first 80 h, and remained nearly constant after 150 h. The A + H treatment emitted markedly a lot of CO2 at the beginning but the flux became lower over time. CO2 evasion from the two low flow treatments exhibited a similar behavior with relatively gentle and steady CO2 emissions, and the N + L treatment released evidently more CO2 than the A + L treatment.
Combined with the result of pCO2 changes (Additional file 1: Figure S2), CO2 uptake could occur by the time when pCO2 concentration dropped below pCO2air. The floating chamber measurement also showed that some CO2 was taken up (CO2 flux < 0) under the high nutrient treatments at the time of 280 h. The rate of CO2 uptake can be calculated from pCO2 and the gas transfer velocity (k600):
\(k_{{\text{H}}}\) is Henry’s constant for CO2 at a given temperature and salinity [49]. Gas transfer velocity (\(k_{600}\)) could be calculated from the initial DIC depletion in the low nutrient treatments assuming that there was little TOC produced in these systems (Fig. 3g, h). Assuming k600 being constant and equivalent under the same flow velocity treatments, k600 was 0.93 and 0.23 m day−1 in the high and low flow velocity treatments, respectively. Using these k600 values and the final pCO2 we can calculate the CO2 flux at the end of the experiment using Eq. (6). Multiplying that flux with the duration of the under-saturated period results in an average total CO2 uptake of 42 and 9.5 μmol in the A + H and A + L treatments, respectively. This is much lower than the observed changes in DIC. Thus, uptake did not significantly contribute to the total C budget of the experiment (Fig. 4).
Discussion
Carbon dynamics
Our results show that flow velocity and nutrient level determine the rate and amount of CO2 evasion, respectively. CO2 evasion depends on both CO2 concentration and the gas transport coefficient (k) in Eq. (6) [50, 51]. With the CO2 evasions under the same flow treatments showing similar tendency over time, flow velocity altered k, which determined the rate of CO2 emission. Meanwhile, treatments with the same nutrient level ended with similar amount of CO2 evasion, suggesting that the nutrient level ultimately dictates the quantity of CO2 emission. Providing that more TOC was generated in the high nutrient treatment, our results confirm that TOC generation reallocate the depletion of inorganic carbon.
Our results also highlight the interaction of the flow and nutrient speeding up the depletion rate of DIC in drainage channels (p = 0.043). However, there was no significant synergistic effect of velocity and nutrients on the final DIC amount remaining in the system (p = 0.40). Flow velocity significantly altered the slope of DIC and CO2 over time, indicating that flow velocity dominated the rate of DIC consumption and CO2 emissions. Simultaneously, flow velocity influences the quantity of DIC consumption as well, possibly by means of regulating on ecosystem metabolism. High flow can intensify the capacity of an ecosystem to store and process carbon [52]. TOC production at high flow velocity could be enhanced by higher light and nutrient availability throughout the water column due to better mixing [53]. Low flow on the other hand may promote sedimentation, thereby improving water clarity which may increase TOC production [33]. Nutrients affect the quantity of both CO2 and DIC by altering the pattern of the inorganic carbon utilization through ecosystem processes (i.e., gross primary production, ecosystem respiration) [54, 55]. Nutrient enrichment may accelerate TOC processing, transformation, and export, potentially altering food-web dynamics and ecosystem stability in the long term [54]. Higher water temperature enhances photosynthesis enzyme activity that favors the biomass growth [56]. In our study, the synergy of both factors cannot be explained by light availability or temperature, because turbidity was low due to lack of sediment and temperature did not differ between treatments (mean water temperature of 25.0 ± 1.0 ℃ within each sampling). Most probable, better mixing at high flow prevented possible local nutrient limitation of photosynthesis in our experiment.
Uncertainty
Effects of C:N ratio on carbon budgets
Our TOC results are sensitive towards the chosen C:N ratio (10:1 in our case). We examined the responsiveness of the C:N ratio to the DIC allocation in our experiment by calculating carbon mass balances for different C:N ratios [C:N = 5, 106:16 (Redfield ratio), 10 (used in this study), and 15] (Additional file 1: Figure S3). Variation of the C:N ratio changed the absolute amount of CO2 emitted from the high nutrient treatments but did not change the general pattern.
Alkalinity dynamics
In a closed system changes in alkalinity only occur by processes including either a solid phase or conversion to a gas, which then escapes to the atmosphere [57]. Beside the definition of the alkalinity as acid neutralizing capacity of a solution titrated to the CO2 equivalence point, the alkalinity can be also described by the equivalent sum of conservative cations (those that do not affect alkalinity) minus the sum of conservative anions:
The changes of alkalinity caused by nitrate and ammonium consumption can account for 0.1–26.2% of the total alkalinity variation. The continuous EC decline during the experiment (Fig. 2) was also observed indicating that not only nitrogen transformation occurred, but there was also ion precipitation inducing alkalinity dropping down. The main ions in the groundwater were SO42−, HCO3− (affects the DIC change), Na+, and Ca2+ [58]. Chemical theory predicts that upon evaporation, half of the bicarbonate in the groundwater will re-equilibrate with the atmosphere releasing CO2 while the other half precipitates, mostly as calcite [11]. Thus, calcite precipitation must have affected our pCO2 calculations and led to alkalinity decrease through Ca2+ removal from the system. Assuming the measured alkalinity changes (153 μmol L−1 in average) were completely caused by calcite precipitation, then there would be 77 μmol L−1 Ca2+ being utilized for CaCO3 formed in each mesocosm. This is roughly consistent with our CO2 emission calculations, because assuming one calcite formed per emitted CO2 would result in 69–168 μmol L−1 Ca2+ being utilized for CaCO3 formed.
Method uncertainties in CO2 flux measurement
We used two methods to quantify CO2 emissions during our experiments: a carbon budget approach and direct flux measurements using floating chambers. Theoretically, the cumulative CO2 flux measured by the chamber should match the cumulative C loss from the water,
where \(S\) is the surface area of the water; \(f\left( t \right)\) is the CO2 emission flux as a function of time; V is the volume of the water, \(c_{0}\) and \(c_{t}\) are the CO2 concentrations at time 0 and t, respectively.
We found that the results of the direct flux measurement and the DIC calculation were not consistent. The flux chamber method resulted in a twenty-time overestimation of the CO2 evasion especially at higher flow (Fig. 4). This is likely because of the artificial turbulence generated by the floating chamber [59, 60]. Deployment of floating chamber in the mesocosm system could cause a large overestimation of the CO2 evasion compared with the one in natural waters [61, 62], which would lead to an unrealistic carbon budget with all carbon removed from the system by outgassing in our carbon budget. Thus, floating chamber measurements cannot be used to quantify CO2 emissions in flume experiments.
Implications and upscaling
Drainage channels receive irrigation water from agriculture and route the nutrient containing groundwater directly into streams. High flow velocity accelerates the CO2 evasion to the atmosphere rapidly (Fig. 5), leaving less opportunity for channel metabolism and processing [32]. Previous research also suggests that CO2 concentrations are largely reduced by intensive primary production in rivers [63]. Thus, nutrient additions have the potential to attenuate CO2 emissions. The formed biomass is transported downstream and could be trapped in lentic parts of the drainage network, where particle sedimentation mainly occurs. Notably, collected particulate carbon needs to be properly buried or dredged. Otherwise methane production is likely to take place in the sediment accumulations. That would largely increase the GHG effect of the drainage network due to the larger global warming potential of methane compared to CO2 [21, 30]. Alternatively, in-stream plankton could potentially be mineralized by heterotrophic respiration [19, 64]. In that case, CO2 would only be temporarily bound and later liberated further downstream.
At a given rate of CO2 emission, the flow velocity determines the required distance until the CO2 concentration in a drainage ditch equilibrates with the atmosphere. In a hypothetical drainage system of infinite length with no biological processes involved, it could be anticipated that the only fate of inorganic carbon is to outgas into atmosphere. Our study suggest that up to 20% of DIC would be converted to CO2 and 58% of the initial DIC would be transported downstream considering no other processes were involved until the pCO2 in the water equilibrates with the atmosphere. Previous study reported large variations of the release rate of atmospheric carbon from rivers to the atmosphere (2–30%) [65]. From field measurements of GHG emissions, Ran et al. [66] concluded that 35% of the carbon exported into the Yellow River network was degassed from the entire watershed during fluvial transport, which is comparable with our estimation.
Irrigation in the study area mainly occurs in March–April (to ensure the water requirement of winter-wheat during the growth period), and October (before wheat sowing). In the case of extreme drought in summer, irrigation is also needed for maize growth in July or August. During other periods the water residence time in the agricultural drainage is quite long, because runoff in irrigated agriculture is mainly related to the intensity of irrigation and rainfall events in comparison to the infiltration capacity of the soil [67]. In NCP, the streamflow has dramatically decreased because of the human activities. Low level of groundwater and large evaporation potential in this semi-arid region often lead to lentic water in drainages [68]. High primary production in the stagnant drainage channels will make them a carbon sink.
The median bicarbonate concentration of aquifer systems in the NCP is approximately 241 mg L−1 based on representative samples from three plain types [36]. Using the conservative assumption that when groundwater reaches the surface, half of the bicarbonate (121 mg L−1) is converted to CO2 [11] which is equivalent to 87 mg L−1 CO2. Annual groundwater net depletion in NCP was estimated as a volume of 8.3 ± 1.1 km3 year−1 [7]. The estimated annual CO2 emissions due to groundwater depletion in the NCP are thus approximately 0.52 ± 0.07 Mt (1 Mt = 1012 g). The irrigation efficiency in this region is about 0.5 as reported earlier [69]. Thus, half of the pumped groundwater reaches the drainage channels. However, we do not know how much of the CO2 is set free already on the fields and which part of it is entering the drainage channels. Furthermore, no biological carbon fixation is considered. This makes our estimate a worst case scenario. Indeed, high CO2 emissions during irrigation the fields have been observed [70]. Agriculture is always an integrative practice with soil management, fertilization, and irrigation. Further studies are needed to understand how much groundwater CO2 is emitted during the agricultural management and how much ends up in the drainage channels.
Groundwater depletion in the NCP contributed a great number of CO2 which is quite close to the groundwater CO2 efflux in US and India (Table 3), and accounted for ~ 5% of the global groundwater extraction. CO2 released from groundwater depletion has not yet been included in the Chinese carbon inventory [71]. A previous study indicates that groundwater loss in the NCP was approximately 50 km3 within 8 years, which is greater than the capacity of China's Three Gorges Dam (39.3 km3), the world’s largest power station [7]. Thus, CO2 emissions from groundwater pumping should be considered in global carbon budgets [72, 73]. This is especially relevant, because groundwater depletion is expected to acceleration in the future [74].
Conclusions
Up to now, carbon dioxide emissions from irrigation drainage networks remain poorly understood. Mesocosm experiments help to unravel the effect of different drivers by experimental manipulation and replications. Here, we applied controlled mesocosm experiments to investigate the impact of flow velocity and nutrients on carbon dynamics in irrigation groundwater entering drainage channels in an intensive agricultural landscape. High nutrients contributed to TOC generation and in turn reduce CO2 evasion on a short term. High flow velocity, on the other hand, promoted rapid CO2 evasion at high nutrient level. Overall, TOC production counteracted CO2 evasion, whereas both of the flow velocity and nutrients stimulate DIC depletion. For a proper quantification of the real CO2 emissions from the entire network, information about flow velocity and residence time of the water in the ditch network as well as information about the fate of organic matter formed in the ditches is necessary. Controlled mesocosm experiments are a useful tool to disentangle the interaction of the various processes involved.
Availability of data and materials
The data of this article can be found online at https://figshare.com/articles/Raw_Data_xlsx/9874394.
References
Giordano M (2009) Global groundwater? Issues and solutions. Annu Rev Environ Resour 34(1):153–178. https://doi.org/10.1146/annurev.environ.030308.100251
Siebert S, Burke J, Faures JM, Frenken K, Hoogeveen J, Döll P, Portmann FT (2010) Groundwater use for irrigation—a global inventory. Hydrol Earth Syst Sci 14(10):1863–1880. https://doi.org/10.5194/hess-14-1863-2010
Famiglietti JS (2014) The global groundwater crisis. Nat Clim Change 4:945. https://doi.org/10.1038/nclimate2425
de Graaf IEM, Gleeson T, van Beek LPH, Sutanudjaja EH, Bierkens MFP (2019) Environmental flow limits to global groundwater pumping. Nature 574(7776):90–94. https://doi.org/10.1038/s41586-019-1594-4
Wang J, Rothausen SGSA, Conway D, Zhang L, Xiong W, Holman IP, Li Y (2012) China’s water–energy nexus: greenhouse-gas emissions from groundwater use for agriculture. Environ Res Lett 7(1):014035. https://doi.org/10.1088/1748-9326/7/1/014035
Zhu X, Li Y, Li M, Pan Y, Shi P (2013) Agricultural irrigation in China. J Soil Water Conserv 68(6):147A-154A. https://doi.org/10.2489/jswc.68.6.147A
Feng W, Zhong M, Lemoine J-M, Biancale R, Hsu H-T, Xia J (2013) Evaluation of groundwater depletion in North China using the Gravity Recovery and Climate Experiment (GRACE) data and ground-based measurements. Water Resour Res 49(4):2110–2118. https://doi.org/10.1002/wrcr.20192
Jahangir MMR, Johnston P, Khalil MI, Hennessy D, Humphreys J, Fenton O, Richards KG (2012) Groundwater: a pathway for terrestrial C and N losses and indirect greenhouse gas emissions. Agric Ecosyst Environ 159:40–48. https://doi.org/10.1016/j.agee.2012.06.015
Macpherson GL (2009) CO2 distribution in groundwater and the impact of groundwater extraction on the global C cycle. Chem Geol 264(1):328–336. https://doi.org/10.1016/j.chemgeo.2009.03.018
Borges AV, Darchambeau F, Lambert T, Bouillon S, Morana C, Brouyère S, Hakoun V, Jurado A, Tseng HC, Descy JP, Roland FAE (2018) Effects of agricultural land use on fluvial carbon dioxide, methane and nitrous oxide concentrations in a large European river, the Meuse (Belgium). Sci Total Environ 610–611:342–355. https://doi.org/10.1016/j.scitotenv.2017.08.047
Wood WW, Hyndman DW (2017) Groundwater depletion: a significant unreported source of atmospheric carbon dioxide. Earth’s Future 5(11):1133–1135. https://doi.org/10.1002/2017EF000586
McGill BM, Hamilton SK, Millar N, Robertson GP (2018) The greenhouse gas cost of agricultural intensification with groundwater irrigation in a Midwest US row cropping system. Glob Change Biol 24(12):5948–5960. https://doi.org/10.1111/gcb.14472
Myhre G, Shindell D, Bréon FM, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque JF, Lee D, Mendoza B, Nakajima T, Robock A, Stephens G, Takemura T, Zhang H (2013) Climate change 2013: the physical science basis: contribution of Working Group I to the Fifth Assessment. Cambridge University Press, Cambridge
Liang LL, Grantz DA, Jenerette GD (2016) Multivariate regulation of soil CO2 and N2O pulse emissions from agricultural soils. Glob Change Biol 22(3):1286–1298. https://doi.org/10.1111/gcb.13130
Houghton RA, House JI, Pongratz J, van der Werf GR, DeFries RS, Hansen MC, Le Quéré C, Ramankutty N (2012) Carbon emissions from land use and land-cover change. Biogeosciences 9(12):5125–5142. https://doi.org/10.5194/bg-9-5125-2012
West TO, Marland G (2003) Net carbon flux from agriculture: carbon emissions, carbon sequestration, crop yield, and land-use change. Biogeochemistry 63(1):73–83. https://doi.org/10.1023/a:1023394024790
Zamanian K, Zarebanadkouki M, Kuzyakov Y (2018) Nitrogen fertilization raises CO2 efflux from inorganic carbon: a global assessment. Glob Change Biol 24(7):2810–2817. https://doi.org/10.1111/gcb.14148
Butman D, Stackpoole S, Stets E, Mcdonald CP, Clow DW, Striegl RG (2016) Aquatic carbon cycling in the conterminous United States and implications for terrestrial carbon accounting. Proc Natl Acad Sci 113(1):58–63. https://doi.org/10.1073/pnas.1512651112
Hotchkiss ER, Hall RO Jr, Sponseller RA, Butman D, Klaminder J, Laudon H, Rosvall M, Karlsson J (2015) Sources of and processes controlling CO2 emissions change with the size of streams and rivers. Nat Geosci 8:696. https://doi.org/10.1038/ngeo2507
Koschorreck M, Downing AS, Hejzlar J, Marcé R, Laas A, Arndt WG, Keller PS, Smolders AJP, van Dijk G, Kosten S (2019) Hidden treasures: human-made aquatic ecosystems harbour unexplored opportunities. Ambio. https://doi.org/10.1007/s13280-019-01199-6
Schrier-Uijl AP, Veraart AJ, Leffelaar PA, Berendse F, Veenendaal EM (2011) Release of CO2 and CH4 from lakes and drainage ditches in temperate wetlands. Biogeochemistry 102(1):265–279. https://doi.org/10.1007/s10533-010-9440-7
Stumm W, Morgan JJ (2012) Aquatic chemistry: chemical equilibria and rates in natural waters, vol 126. Wiley, New York
Duvert C, Bossa M, Tyler KJ, Wynn JG, Munksgaard NC, Bird MI, Setterfield SA, Hutley LB (2019) Groundwater-derived DIC and carbonate buffering enhance fluvial CO2 evasion in two Australian Tropical Rivers. J Geophys Res Biogeosci 124(2):312–327. https://doi.org/10.1029/2018jg004912
Cole JJ, Prairie YT, Caraco NF, Mcdowell WH, Tranvik LJ, Striegl RG, Duarte CM, Kortelainen P, Downing JA, Middelburg JJ (2007) Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10(1):172–185. https://doi.org/10.1007/s10021-006-9013-8
Borges AV, Darchambeau F, Teodoru CR, Marwick TR, Tamooh F, Geeraert N, Omengo FO, Guerin F, Lambert T, Morana C, Okuku E, Bouillon S (2015) Globally significant greenhouse-gas emissions from African inland waters. Nat Geosci 8(8):637–642. https://doi.org/10.1038/ngeo2486
Raymond PA, Hartmann J, Lauerwald R, Sobek S, McDonald C, Hoover M, Butman D, Striegl R, Mayorga E, Humborg C, Kortelainen P, Durr H, Meybeck M, Ciais P, Guth P (2013) Global carbon dioxide emissions from inland waters. Nature 503(7476):355–359. https://doi.org/10.1038/nature12760
Halbedel S, Koschorreck M (2013) Regulation of CO2 emissions from temperate streams and reservoirs. Biogeosciences 10(11):7539–7551. https://doi.org/10.5194/bg-10-7539-2013
Borges AV, Darchambeau F, Lambert T, Morana C, Allen GH, Tambwe E, Toengaho Sembaito A, Mambo T, Nlandu Wabakhangazi J, Descy JP, Teodoru CR, Bouillon S (2019) Variations in dissolved greenhouse gases (CO2, CH4, N2O) in the Congo River network overwhelmingly driven by fluvial-wetland connectivity. Biogeosciences 16(19):3801–3834. https://doi.org/10.5194/bg-16-3801-2019
Dinsmore KJ, Billett MF, Dyson KE (2013) Temperature and precipitation drive temporal variability in aquatic carbon and GHG concentrations and fluxes in a peatland catchment. Glob Change Biol 19(7):2133–2148. https://doi.org/10.1111/gcb.12209
Gómez-Gener L, Obrador B, von Schiller D, Marcé R, Casas-Ruiz JP, Proia L, Acuña V, Catalán N, Muñoz I, Koschorreck M (2015) Hot spots for carbon emissions from Mediterranean fluvial networks during summer drought. Biogeochemistry 125(3):409–426. https://doi.org/10.1007/s10533-015-0139-7
Liu S, Lu XX, Xia X, Yang X, Ran L (2017) Hydrological and geomorphological control on CO2 outgassing from low-gradient large rivers: an example of the Yangtze River system. J Hydrol 550:26–41. https://doi.org/10.1016/j.jhydrol.2017.04.044
Liu S, Raymond PA (2018) Hydrologic controls on pCO2 and CO2 efflux in US streams and rivers. Limnol Oceanogr Lett 3(6):428–435. https://doi.org/10.1002/lol2.10095
Crawford JT, Loken LC, Stanley EH, Stets EG, Dornblaser MM, Striegl RG (2016) Basin scale controls on CO2 and CH4 emissions from the Upper Mississippi River. Geophys Res Lett 43(5):1973–1979. https://doi.org/10.1002/2015GL067599
Zhao X, Li F, Ai Z, Li J, Gu C (2018) Stable isotope evidences for identifying crop water uptake in a typical winter wheat–summer maize rotation field in the North China Plain. Sci Total Environ 618:121–131. https://doi.org/10.1016/j.scitotenv.2017.10.315
Zhou Z, Zhang G, Yan M, Wang J (2012) Spatial variability of the shallow groundwater level and its chemistry characteristics in the low plain around the Bohai Sea, North China. Environ Monit Assess 184(6):3697–3710. https://doi.org/10.1007/s10661-011-2217-1
Chen Z, Nie Z, Zhang Z, Qi J, Nan Y (2005) Isotopes and sustainability of ground water resources, North China Plain. Groundwater 43(4):485–493. https://doi.org/10.1111/j.1745-6584.2005.0038.x
Zhang Y, Li F, Li J, Liu Q, Tu C, Suzuki Y, Huang C (2015) Spatial distribution, potential sources, and risk assessment of trace metals of groundwater in the North China Plain. Hum Ecol Risk Assess 21(3):726–743. https://doi.org/10.1080/10807039.2014.921533
Shao J, Ling Y, Zhang Z (2013) Groundwater flow simulation and its application in groundwater resource evaluation in the North China Plain, China. Acta Geol Sin 87(1):243–253. https://doi.org/10.1111/1755-6724.12045
Petersen JE, Kemp WM, Bartleson R, Boynton WR, Chen C-C, Cornwell JC, Gardner RH, Hinkle DC, Houde ED, Malone TC (2003) Multiscale experiments in coastal ecology: improving realism and advancing theory. Bioscience 53(12):1181–1197. https://doi.org/10.1641/0006-3568(2003)053[1181:MEICEI]2.0.CO;2
Liboriussen L, Landkildehus F, Meerhoff M, Bramm ME, Søndergaard M, Christoffersen K, Richardson K, Søndergaard M, Lauridsen TL, Jeppesen E (2005) Global warming: design of a flow-through Shallow Lake mesocosm climate experiment. Limnol Oceanogr Methods 3(1):1–9. https://doi.org/10.4319/lom.2005.3.1
Parkhurst DL, Appelo C (2013) Description of input and examples for PHREEQC version 3: a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geological Survey
Li J, Li F, Liu Q, Zhang Y (2014) Trace metal in surface water and groundwater and its transfer in a Yellow River alluvial fan: evidence from isotopes and hydrochemistry. Sci Total Environ 472:979–988. https://doi.org/10.1016/j.scitotenv.2013.11.120
Hecky RE, Campbell P, Hendzel LL (1993) The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol Oceanogr 38(4):709–724. https://doi.org/10.4319/lo.1993.38.4.0709
They NH, Amado AM, Cotner JB (2017) Redfield ratios in inland waters: higher biological control of C:N:P ratios in tropical semi-arid high water residence time lakes. Front Microbiol 8:1505–1505. https://doi.org/10.3389/fmicb.2017.01505
Stubbins A (2016) A carbon for every nitrogen. Proc Natl Acad Sci 113(39):10736–10738. https://doi.org/10.1073/pnas.1612995113
Zeebe RE, Wolf-Gladrow D (2001) CO2 in seawater: equilibrium, kinetics, isotopes, vol 65. Gulf Professional Publishing, Amsterdam
R Core Team (2019) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Lewis E, Wallace D, Allison LJ (1998) Program developed for CO2 system calculations. Brookhaven National Lab., Dept. of Applied Science, Upton, NY (United States)
Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar Chem 2(3):203–215. https://doi.org/10.1016/0304-4203(74)90015-2
Alin SR, de Fátima FLRM, Salimon CI, Richey JE, Holtgrieve GW, Krusche AV, Snidvongs A (2011) Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J Geophys Res Biogeosci. https://doi.org/10.1029/2010JG001398
Raymond PA, Zappa CJ, Butman D, Bott TL, Potter J, Mulholland P, Laursen AE, McDowell WH, Newbold D (2012) Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers. Limnol Oceanogr Fluids Environ 2(1):41–53. https://doi.org/10.1215/21573689-1597669
Aristi I, Arroita M, Larrañaga A, Ponsatí L, Sabater S, von Schiller D, Elosegi A, Acuña V (2014) Flow regulation by dams affects ecosystem metabolism in Mediterranean rivers. Freshw Biol 59(9):1816–1829. https://doi.org/10.1111/fwb.12385
Moss BR (2009) Ecology of fresh waters: man and medium, past to future. Wiley, New York
Benstead JP, Rosemond AD, Cross WF, Wallace JB, Eggert SL, Suberkropp K, Gulis V, Greenwood JL, Tant CJ (2009) Nutrient enrichment alters storage and fluxes of detritus in a headwater stream ecosystem. Ecology 90(9):2556–2566. https://doi.org/10.1890/08-0862.1
Williamson TJ, Cross WF, Benstead JP, Gíslason GM, Hood JM, Huryn AD, Johnson PW, Welter JR (2016) Warming alters coupled carbon and nutrient cycles in experimental streams. Glob Change Biol 22(6):2152–2164. https://doi.org/10.1111/gcb.13205
Hall RO Jr, Yackulic CB, Kennedy TA, Yard MD, Rosi-Marshall EJ, Voichick N, Behn KE (2015) Turbidity, light, temperature, and hydropeaking control primary productivity in the Colorado River, Grand Canyon. Limnol Oceanogr 60(2):512–526. https://doi.org/10.1002/lno.10031
Koschorreck M, Tittel J (2007) Natural alkalinity generation in neutral lakes affected by acid mine drainage. J Environ Qual 36(4):1163–1171. https://doi.org/10.2134/jeq2006.0354
Zhang F, Li F, Li J, Shuai S, Cai W, Chang C (2013) Hydrochemical characteristics of surface water in main rivers of the irrigation districts in the downstream of Yellow River. Acta Autom Sin 21(4):487–493. https://doi.org/10.3724/SP.J.1011.2013.00487
Lorke A, Bodmer P, Noss C, Alshboul Z, Koschorreck M, Somlaihaase C, Bastviken D, Flury S, Mcginnis DF, Maeck A (2015) Technical note: drifting versus anchored flux chambers for measuring greenhouse gas emissions from running waters. Biogeosciences 12(23):7013–7024. https://doi.org/10.5194/bg-12-7013-2015
Raymond PA, Cole JJ (2001) Gas exchange in rivers and Estuaries: choosing a gas transfer velocity. Estuaries 24(2):312–317. https://doi.org/10.2307/1352954
Marino R, Howarth RW (1993) Atmospheric oxygen exchange in the Hudson River: dome measurements and comparison with other natural waters. Estuaries 16(3):433–445. https://doi.org/10.2307/1352591
Vachon D, Prairie YT, Cole JJ (2010) The relationship between near-surface turbulence and gas transfer velocity in freshwater systems and its implications for floating chamber measurements of gas exchange. Limnol Oceanogr 55(4):1723–1732. https://doi.org/10.4319/lo.2010.55.4.1723
Houser JN, Bartsch LA, Richardson WB, Rogala JT, Sullivan JF (2015) Ecosystem metabolism and nutrient dynamics in the main channel and backwaters of the Upper Mississippi River. Freshw Biol 60(9):1863–1879. https://doi.org/10.1111/fwb.12617
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37(1):130–137. https://doi.org/10.1139/f80-017
Li S, Lu XX, He M, Zhou Y, Li L, Ziegler AD (2012) Daily CO2 partial pressure and CO2 outgassing in the upper Yangtze River basin: a case study of the Longchuan River, China. J Hydrol 466:141–150. https://doi.org/10.1016/j.jhydrol.2012.08.011
Ran L, Lu XX, Yang H, Li L, Yu R, Sun H, Han J (2015) CO2 outgassing from the Yellow River network and its implications for riverine carbon cycle. J Geophys Res Biogeosci 120(7):1334–1347. https://doi.org/10.1002/2015jg002982
Foster S, Pulido-Bosch A, Vallejos Á, Molina L, Llop A, MacDonald AM (2018) Impact of irrigated agriculture on groundwater-recharge salinity: a major sustainability concern in semi-arid regions. Hydrogeol J 26(8):2781–2791. https://doi.org/10.1007/s10040-018-1830-2
Varis O, Vakkilainen P (2001) China’s 8 challenges to water resources management in the first quarter of the 21st century. Geomorphology 41(2):93–104. https://doi.org/10.1016/S0169-555X(01)00107-6
Qu SS, Zhu ZZ, Qiu N, Wang WP (2012) The impact of irrigation diverting from Yellow River water on regional water cycle in Shandong Province. Appl Mech Mater 212–213:514–517. https://doi.org/10.4028/www.scientific.net/AMM.212-213.514
Tang J, Wang J, Li Z, Wang S, Qu Y (2018) Effects of irrigation regime and nitrogen fertilizer management on CH4, N2O and CO2 emissions from saline-alkaline paddy fields in Northeast China. Sustainability 10(2):475. https://doi.org/10.3390/su10020475
Shan Y, Guan D, Zheng H, Ou J, Li Y, Meng J, Mi Z, Liu Z, Zhang Q (2018) China CO2 emission accounts 1997–2015. Sci Data 5:170201. https://doi.org/10.1038/sdata.2017.201
Currell MJ, Cartwright I, Bradley DC, Han D (2010) Recharge history and controls on groundwater quality in the Yuncheng Basin, North China. J Hydrol 385(1):216–229. https://doi.org/10.1016/j.jhydrol.2010.02.022
Mishra V, Asoka A, Vatta K, Lall U (2018) Groundwater depletion and associated CO2 emissions in India. Earth’s Future 6(12):1672–1681. https://doi.org/10.1029/2018ef000939
Zhao Q, Zhang B, Yao Y, Wu W, Meng G, Chen Q (2019) Geodetic and hydrological measurements reveal the recent acceleration of groundwater depletion in North China Plain. J Hydrol 575:1065–1072. https://doi.org/10.1016/j.jhydrol.2019.06.016
Qiu GY, Zhang X, Yu X, Zou Z (2018) The increasing effects in energy and GHG emission caused by groundwater level declines in North China’s main food production plain. Agric Water Manag 203:138–150. https://doi.org/10.1016/j.agwat.2018.03.003
Zhang X (2019) Multiple cropping system expansion: increasing agricultural greenhouse gas emissions in the north China plain and neighboring regions. Sustainability 11(14):3941. https://doi.org/10.3390/su11143941
Cui Y, Zhang W, Wang C, Streets DG, Xu Y, Du M, Lin J (2019) Spatiotemporal dynamics of CO2 emissions from central heating supply in the North China Plain over 2012–2016 due to natural gas usage. Appl Energy 241:245–256. https://doi.org/10.1016/j.apenergy.2019.03.060
Liu M, Song Y, Yao H, Kang Y, Li M, Huang X, Hu M (2015) Estimating emissions from agricultural fires in the North China Plain based on MODIS fire radiative power. Atmos Environ 112:326–334. https://doi.org/10.1016/j.atmosenv.2015.04.058
Acknowledgements
We thank colleagues from Department of Lake Research in Helmholtz Center for Environmental Research for valuable discussion.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) No. 41771292. P. Leng is supported by the CAS-DAAD Joint Fellowship Program for Doctoral Students.
Author information
Authors and Affiliations
Contributions
PL and FL designed the mesocosm experiment. PL, KD, and ZL were involved in performing the mesocosm experiment and collecting the samples. PL, CG, and MK analyzed and interpreted the data. PL drafted the manuscript. MK contributed to the study design and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Supplementary materials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leng, P., Li, F., Du, K. et al. Flow velocity and nutrients affect CO2 emissions from agricultural drainage channels in the North China Plain. Environ Sci Eur 32, 146 (2020). https://doi.org/10.1186/s12302-020-00426-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-020-00426-2