Abstract
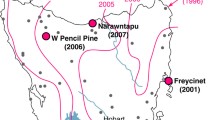
Telomeres protect chromosomes from degradation during cellular replication. In humans, it is well-documented that excessive telomere degradation is one mechanism by which cells can become cancerous. Increasing evidence from wildlife studies suggests that telomere length is positively correlated with survival and health and negatively correlated with disease infection intensity. The recently emerged devil facial tumor disease (DFTD) has led to dramatic and rapid population declines of the Tasmanian devil throughout its geographic range. Here, we tested the hypothesis that susceptibility to DFTD is negatively correlated with telomere length in devils across three populations with different infection histories. Our findings suggest telomere length is correlated with DFTD resistance in three ways. First, devils from a population with the slowest recorded increase in DFTD prevalence (West Pencil Pine) have significantly longer telomeres than those from two populations with rapid and exponential increases in prevalence (Freycinet and Narawantapu). Second, using extensive mark-recapture data obtained from a long-term demographic study, we found that individuals with relatively long telomeres tend to be infected at a significantly later age than those with shorter telomeres. Third, a hazard model showed devils with longer telomeres tended to become infected at a lower rate than those with shorter telomeres. This research provides a rare study of telomere length variation and its association with disease in a wildlife population. Our results suggest that telomere length may be a reliable marker of susceptibility to DFTD and assist with future management of this endangered species.





Similar content being viewed by others
References
Angelier F, Vleck CM, Holberton RL, Marra PP (2013) Telomere length, non-breeding habitat and return rate in male American redstarts. Functional Ecology 27: 342–350
Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S (2015) Chronic infection. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347: 436–8
Barton K (2016) MuMIn: Multi-Model Inference. R package version 1.15.6. URL https://CRAN.R-project.org/package=MuMIn
Beirne C, Delahay R, Hares M, Young A (2014) Age-related declines and disease-associated variation in immune cell telomere length in a wild mammal. PLoS One 9: e108964.
Bender HS, Murchison EP, Pickett HA, Deakin JE, Strong MA, Conlan C, McMillan DA, Neumann AA, Greider CW, Hannon GJ, Reddel RR, Graves JAM (2012) Extreme telomere length dimorphism in the Tasmanian devil and related marsupials suggests parental control of telomere length. PLoS One 7: e46195
Blackburn EH (1991) Structure and function of telomeres. Nature 350: 569–573
Campisi J, Kim S, Lim C, Rubio M (2001) Cellular senescence, cancer and aging: the telomere connection. Experimental Gerontology 36: 1619–1637
Cattan V, Mercier N, Gardner JP, Regnault V, Labat C, Maki-Jouppila J, Nzietchueng R, Benetos A, Kimura M, Aviv A, Lacolley P (2008) Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radical Biology and Medicine 8: 1592-8.
Chan SRWL, and Blackburn EH, (2004) Telomeres and telomerase. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 109–21
Constantini D, Marasco V, Moller AP (2011) A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. Journal of Comparative Physiology B 181: 447–56
Coppé JP, Kauser K, Campisi J, Beausejour CM (2006) Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. Journal of Biological Chemistry28: 29568–29574
Ducray C, Pommier JP, Martins L, Boussin FD, Sabatier L (1999) Telomere dynamics, end-to-end fusions and telomerase activation during the human fibroblast immortalization process. Oncogene 18(29): 4211-4223
Ganem NJ and Pellman D (2007) Limiting the proliferation of polyploid cells. Cell131:437–440
Glaser R and Kiecolt-glaser JK (2005) Stress-induced immune dysfunction: implications for health.Nature Reviews Immunology 5: 243-251
Glei D, Goldman N, Risques RA, Rehkopf DH, Dow WH, Rosero-Bixby L, Weinstein M (2016) Predicting Survival from telomere length versus conventional
Gomes NM (2011) Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 10: 761-8
Hackett JA and Greider CW (2002) Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene 21: 619–626
Hamede R, Lachish S, Belov K, Woods G, Kreiss A, Pearse AM, Lazenby B, Jones M, McCallum H (2012) Reduced effect of Tasmanian devil facial tumor disease at the disease front. Conservation Biology 26: 124–34
Hamede R, McCallum H, Jones, M (2013). Biting injuries and transmission of Tasmanian devil
Hamede R, Pearse AM, Swift K, Barmuta LA, Murchison EP, Jones ME (2015) Transmissible cancer in Tasmanian devils: localized lineage replacement and host population response. Proceedings of the Royal Society B: Biological Sciences282: 20151468
Haussmann MF, Winkler DW, Vleck CM (2005) Longer telomeres associated with higher survival in birds. Biology Letters 1: 212–4
Hawkins CE, Baars C, Hesterman H, Hocking GJ, Jones ME, Lazenby B, Mann D, Mooney N, Pemberton D, Pyecroft S, Restani M, Wiersma J (2006) Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation 131: 307–324
Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. Proceedings of the National Academy of Sciences, USA 109: 1743–8
Joeng KS, Song EJ, Lee K, Lee J (2004) Long lifespan in worms with long telomeric DNA. Nature Genetics 36: 607–611
Jones ME, Cockburn A, Hamede R, Hawkins C, Hesterman H, Lachish S, Mann D, McCallum H, Pemberton D (2008) Life history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences, USA 105: 10023–10027
Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proceedings of the National Academy of Sciences, USA98: 12072–7
Kwon YM, Stammnitz MR, Wang J, Swift K, Knowles GW, Pye RJ, Kreiss A, Peck S, Fox S, Pemberton D, Jones ME, Hamede R, Murchison MP (2018) Tasman-PCR: a genetic diagnostic assay for Tasmanian devil facial tumour diseases. Royal Society Open Interface5: 180070.
Ma, H, Zhou Z, Wei S, Liu Z, Pooley K, Dunning A, Svenson U, Roos G, Hosgood III D, Shen M, Wei Q (2011) Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One 6: e20466
Martin-Ruiz C, Baird D, Roger L, Boukamp P, Krunic D, Cawthon R, Dokter M, Harst PVD, Bekaert S, Meyer TD, Roos G, Svenson U, Codd V, Samani NJ, McGlynn L, Shiels PG, Pooley KA, Dunning AM, Cooper R, Wong A, Kingston A, Zglinicki TV (2015) Reproducibility of telomere length assessment: an international collaborative study. International Journal of Epidemiology 44: 1673-83
McCallum H, Jones ME, Hawkins C, Hamede R, Lachish S, Sinn D, Beeton N, Lazenby B (2009) Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology 90: 3379–3392
Miller W, Hayes VM, Ratan A, et al (2011) Genetic diversity and population structure of the endangered marsupial Sarcophilus harrisii (Tasmanian devil). Proceedings of the National Academy of Sciences, USA 108:12348–12353
Mizutani Y, Tomita N, Niizuma Y, Yoda K (2013) Environmental perturbations influence telomere dynamics in long-lived birds in their natural habitat. Biology Letters9: 20130511
Monaghan P and Haussmann MF (2006) Do telomere dynamics link lifestyle and lifespan? Trends in Ecology and Evolution 21: 47–53
Pearse AM, Swift K, Hodson P, Hua B, McCallum H, Pyecroft S, Taylor R, Eldridge MDB, Belov K (2012) Evolution in a transmissible cancer: A study of the chromosomal changes in devil facial tumor (DFT) as it spreads through the wild Tasmanian devil population. Cancer Genetics 205:101–102
Pye R, Hamede R, Siddle HV, Caldwell A, Knowles GW, Swift K, Kreiss A, Jones ME, Lyons AB, Woods GM (2016) Demonstration of immune responses against devil facial tumour disease in wild Tasmanian devils. Biology Letters 12: 20160553
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Richter T and von Zglinicki T (2007) A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Experimental Gerontology 42: 1039–42
Romano GH, Harari Y, Yehuda T, Podhorzer A, Rubinstein L, Shamir R, Gottlieb A, Silberberg Y, Pe’er D, Ruppin E, Sharan R, Kupiec M (2013) Environmental stresses disrupt telomere length homeostasis. PLoS Genetics 9: e1003721
Siddle HV, Kreiss A, Tovar C, Yuen CK, Cheng Y, Belov K, Swift K, Pearse AM, Hamede R, Jones ME, Skjodt K, Woods GM, Kaufman J (2013) Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proceedings of the National Academy of Sciences, USA 110: 5103–8
Symonds MRE and Moussalli A (2010) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology65: 13–21
Therneau T (2015) A Package for Survival Analysis in S. version 2.38 URL https://CRAN.R-project.org/package=survival
Ujvari B, Pearse AM, Taylor R, Pyecroft S, Flanagan C, Gombert S, Papenfuss AT, Madsen T, Belov K (2012) Telomere dynamics and homeostasis in a transmissible cancer. PLoS One7: e44085
Wang N-P (2012) Telomeres and immune competency. Current Opinion in Immunology24: 470-475
Webb CJ, Wu Y, Zakian VA (2013) DNA repair at telomeres: Keeping the ends intact. Cold Spring Harbor Perspectives in Biology5:a012666
Weischer M, Nordestgaard, BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Bojesen SE (2013) Short telomere length, cancer survival, and cancer risk in 47102 individuals. Journal of the National Cancer Institute105: 459–68
Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidemiology, Biomarkers, and Prevention20: 1238–50
Zhang C, Chen X, Ying Zhou LL, Wang C, Hou S (2015) The Association between telomere length and cancer prognosis: evidence from a meta-analysis. PLoS One 10: e0133174
Acknowledgements
This work was funded under NSF Grant DEB 1316549 and NIH Grant R01-GM126563 to AS and MJ as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases program. Animal use was approved under IACUC protocol ASAF#04392 at Washington State University. Fieldwork was funded by grants from the Australian Research Council to MJ (A00000162, LP0561120, LP0989613, DP110102656), several Eric Guiler Tasmanian devil grants, through the Save the Tasmanian devil Appeal of the University of Tasmania Foundation to R.H and M.J., and grants from the Ian Potter Foundation, the Australian Academy of Science (Margaret Middleton Fund), Estate of WV Scott, the National Geographic Society, the Mohammed bin Zayed Conservation Fund, and the Holsworth Wildlife Trust to MJ. MJ received support from an ARC Future Fellowship (FT100100250) and a Fulbright Tasmania Senior Scholarship, and RH from an ARC DECRA (DE170101116). We are grateful to M & C Walsh from Discovery Holiday Parks at Cradle Mountain, and the Tasmanian Parks and Wildlife Service at Freycinet, Cradle Mountain and Narawntapu for providing accommodation and logistic support during fieldwork and a large number of volunteers who helped to collect data. Forico Pty Ltd. provided land access and logistic support during fieldwork. We thank Jesse Brunner for assistance with hazard model analyses. We also thank Austin Patton, Alexandra Fraik, Lauren Ricci, Omar Cornejo and Paul Hohenlohe for rich discussions that improved the quality of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, L.E., Jones, M.E., Hamede, R. et al. Telomere Length is a Susceptibility Marker for Tasmanian Devil Facial Tumor Disease. EcoHealth 17, 280–291 (2020). https://doi.org/10.1007/s10393-020-01491-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-020-01491-y