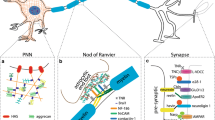
Abstract
The extracellular matrix (ECM) is a fundamental component of biological tissues. The ECM in the central nervous system (CNS) is unique in both composition and function. Functions such as learning, memory, synaptogenesis, and plasticity are regulated by numerous ECM molecules. The neural ECM acts as a non-specific physical barrier that modulates neuronal plasticity and axon regeneration. There are two specialized types of ECM in the CNS, diffuse perisynaptic ECM and condensed ECM, which selectively surround the perikaryon and initial part of dendritic trees in subtypes of neurons, forming perineuronal nets. This review presents the current knowledge about the role of important neuronal ECM molecules in maintaining the basic functions of a neuron, including electrogenesis and the ability to form neural circuits. The review mainly focuses on the role of ECM components that participate in the control of key events such as cell survival, axonal growth, and synaptic remodeling. Particular attention is drawn to the numerous molecular partners of the main ECM components. These regulatory molecules are integrated into the cell membrane or disposed into the matrix itself in solid or soluble form. The interaction of the main matrix components with molecular partners seems essential in molecular mechanisms controlling neuronal functions. Special attention is paid to the chondroitin sulfate proteoglycan 4, type 1 transmembrane protein, neural-glial antigen 2 (NG2/CSPG4), whose cleaved extracellular domain is such a molecular partner that it not only acts directly on neural and vascular cells, but also exerts its influence indirectly by binding to resident ECM molecules.

Similar content being viewed by others
References
Allen NJ, Bennett ML, Foo LC et al (2012) Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486:410–414
Anderson MA, Burda JE, Ren Y et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195
Andrews MR, Czvitkovich S, Dassie E et al (2009) α9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci 29:5546–5557
Anlar B, Gunel-Ozcan A (2012) Tenascin-R: role in the central nervous system. Int J Biochem Cell Biol 44:1385–1389
Apostolova I, Irintchev A, Schachner M (2006) Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J Neurosci 26:7849–7859
Arranz AM, Perkins KL, Irie F et al (2014) Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci 34:6164–6176
Asher RA, Morgenstern DA, Properzi F et al (2005) Two separate metalloproteinase activities are responsible for the shedding and processing of the NG2 proteoglycan in vitro. Mol Cell Neurosci 29:82–96
Azizi M, Farahmandghavi F, Joghataei MT et al (2020) ChABC-loaded PLGA nanoparticles: a comprehensive study on biocompatibility, functional recovery, and axonal regeneration in animal model of spinal cord injury. Int J Pharm 577:119037
Barritt DS, Pearn MT, Zisch AH et al (2000) The multi-PDZ domain protein MUPP1 is a cytoplasmic ligand for the membrane-spanning proteoglycan NG2. J Cell Biochem 79:213–224
Bartus K, James ND, Bosch KD, Bradbury EJ (2012) Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol 235:5–17
Becker T, Anliker B, Becker CG et al (2000) Tenascin-R inhibits regrowth of optic fibers in vitro and persists in the optic nerve of mice after injury. Glia 29:330–346
Bekku Y, Oohashi T (2019) Under the ECM dome: the physiological role of the perinodal extracellular matrix as an ion diffusion barrier. In: Myelin, vol 8. Springer, Berlin, pp 107–122
Bekku Y, Su W-D, Hirakawa S et al (2003) Molecular cloning of Bral2, a novel brain-specific link protein, and immunohistochemical colocalization with brevican in perineuronal nets☆. Mol Cell Neurosci 24:148–159
Bekku Y, Vargová L, Goto Y et al (2010) Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci 30:3113–3123
Bekku Y, Saito M, Moser M et al (2012) Bral2 is indispensable for the proper localization of brevican and the structural integrity of the perineuronal net in the brainstem and cerebellum. J Comp Neurol 520:1721–1736
Bell SC, Pringle JH, Taylor DJ, Malak TM (1999) Alternatively spliced tenascin-C mRNA isoforms in human fetal membranes. Mol Hum Reprod 5:1066–1076
Beurdeley M, Spatazza J, Lee HHC et al (2012) Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 32:9429–9437
Bijata M, Labus J, Guseva D et al (2017) Synaptic remodeling depends on signaling between serotonin receptors and the extracellular matrix. Cell Rep 19:1767–1782
Blosa M, Sonntag M, Jäger C et al (2015) The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of held. J Physiol 593:4341–4360
Blümcke I, Eggli P, Celio MR (1995) Relationship between astrocytic processes and “perineuronal nets” in rat neocortex. Glia 15:131–140
Bosiacki M, Gąssowska-Dobrowolska M, Kojder K et al (2019) Perineuronal nets and their role in synaptic homeostasis. Int J Mol Sci 20:4108
Bourguignon LYW, Gilad E, Peyrollier K et al (2007) Hyaluronan-CD44 interaction stimulates Rac1 signaling and PKNγ kinase activation leading to cytoskeleton function and cell migration in astrocytes. J Neurochem 101:1002–1017
Bradbury EJ, Moon LDF, Popat RJ et al (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640
Brown JM, Xia J, Zhuang B et al (2012) A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci 109:4768–4773
Brückner G, Seeger G, Brauer K et al (1994) Cortical areas are revealed by distribution patterns of proteoglycan components and parvalbumin in the Mongolian gerbil and rat. Brain Res 658:67–86
Brückner G, Grosche J, Schmidt S et al (2000) Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol 428:616–629
Bu J, Akhtar N, Nishiyama A (2001) Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia 34:296–310
Bukalo O, Schachner M, Dityatev A (2001) Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104:359–369
Burket JA, Urbano MR, Deutsch SI (2017) Sugarcoated perineuronal nets regulate “GABAergic” transmission: Bittersweet hypothesis in Autism Spectrum Disorder. Clin Neuropharmacol 40:120–130
Busch SA, Horn KP, Cuascut FX et al (2010) Adult NG2 + cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci 30:255–265
Cabungcal J-H, Steullet P, Morishita H et al (2013) Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci 110:9130–9135
Carulli D, Rhodes KE, Brown DJ et al (2006) Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol 494:559–577
Carulli D, Pizzorusso T, Kwok JCF et al (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133:2331–2347
Carulli D, Foscarin S, Faralli A et al (2013) Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci 57:10–22
Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L (1998) Perineuronal nets: past and present. Trends Neurosci 21:510–515
Chang MC, Park JM, Pelkey KA et al (2010) Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci 13:1090
Cheah M, Andrews MR, Chew DJ et al (2016) Expression of an activated integrin promotes long-distance sensory axon regeneration in the spinal cord. J Neurosci 36:7283–7297
Chen J, Lee HJ, Jakovcevski I et al (2010) The extracellular matrix glycoprotein tenascin-C is beneficial for spinal cord regeneration. Mol Ther 18:1769–1777
Chiquet-Ehrismann R (1991) Anti-adhesive molecules of the extracellular matrix. Curr Opin Cell Biol 3:800–804
Christopherson KS, Ullian EM, Stokes CCA et al (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120:421–433
Chung W-S, Lee E (2019) Glial control of synapse number in healthy and diseased brain. Front Cell Neurosci 13:42
Chung CY, Zardi L, Erickson HP (1995) Binding of tenascin-C to soluble fibronectin and matrix fibrils. J Biol Chem 270:29012–29017
de Castro Jr R, Tajrishi R, Claros J, Stallcup WB (2005) Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol 192:299–309
De Laporte L, Rice JJ, Tortelli F, Hubbell JA (2013) Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS ONE 8:156
De Luca C, Colangelo AM, Virtuoso A et al (2020) Neurons, glia, extracellular matrix and neurovascular unit: a systems biology approach to the complexity of synaptic plasticity in health and disease. Int J Mol Sci 21:1539
De Winter F, Kwok JCF, Fawcett JW et al (2016) The chemorepulsive protein semaphorin 3A and perineuronal net-mediated plasticity. Neural Plast 20:256
Deckner M, Lindholm T, Cullheim S, Risling M (2000) Differential expression of tenascin-C, tenascin-R, tenascin/J1, and tenascin-X in spinal cord scar tissue and in the olfactory system. Exp Neurol 166:350–362
Deepa SS, Carulli D, Galtrey C et al (2006) Composition of perineuronal net extracellular matrix in rat brain a different disaccharide composition for the net-associated proteoglycans. J Biol Chem 281:17789–17800
Derouiche A, Härtig W, Brauer K, Brückner G (1996) Spatial relationship of lectin-labelled extracellular matrix and glutamine synthetase-immunoreactive astrocytes in rat cortical forebrain regions. J Anat 189:363
Dick G, Tan CL, Alves JN et al (2013) Semaphorin 3A binds to the perineuronal nets via chondroitin sulfate type E motifs in rodent brains. J Biol Chem 288:27384–27395
Dickendesher TL, Baldwin KT, Mironova YA et al (2012) NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 15:703
Dityatev A, Rusakov DA (2011) Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 21:353–359
Dityatev A, Schachner M (2003) Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4:456–468
Dityatev A, Schachner M (2006) The extracellular matrix and synapses. Cell Tissue Res 326:647–654
Dityatev A, Brückner G, Dityateva G et al (2007) Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol 67:570–588
Dityatev A, Seidenbecher CI, Schachner M (2010) Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci 33:503–512
Djerbal L, Lortat-Jacob H, Kwok JCF (2017) Chondroitin sulfates and their binding molecules in the central nervous system. Glycoconj J 34:363–376
Dobbertin A, Czvitkovich S, Theocharidis U et al (2010) Analysis of combinatorial variability reveals selective accumulation of the fibronectin type III domains B and D of tenascin-C in injured brain. Exp Neurol 225:60–73
Donnelly EM, Strappe PM, McGinley LM et al (2010) Lentiviral vector-mediated knockdown of the neuroglycan 2 proteoglycan or expression of neurotrophin-3 promotes neurite outgrowth in a cell culture model of the glial scar. J Gene Med 12:863–872
Dou C-L, Levine JM (1994) Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci 14:7616–7628
Duan Y, Giger RJ (2010) A new role for RPTPσ in spinal cord injury: signaling chondroitin sulfate proteoglycan inhibition. Sci Signal 3:pe6-pe6
Dyck SM, Karimi-Abdolrezaee S (2015) Chondroitin sulfate proteoglycans: key modulators in the developing and pathologic central nervous system. Exp Neurol 269:169–187
Dzyubenko E, Gottschling C, Faissner A (2016) Neuron-glia interactions in neural plasticity: contributions of neural extracellular matrix and perineuronal nets. Neural Plast 16:258–263
Evers MR, Salmen B, Bukalo O et al (2002) Impairment of L-type Ca2 + channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J Neurosci 22:7177–7194
Faissner A (1997) The tenascin gene family in axon growth and guidance. Cell Tissue Res 290:331–341
Faissner A, Pyka M, Geissler M et al (2010) Contributions of astrocytes to synapse formation and maturation—potential functions of the perisynaptic extracellular matrix. Brain Res Rev 63:26–38
Favuzzi E, Marques-Smith A, Deogracias R et al (2017) Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 95:639–655
Fawcett JW (2017) An integrin approach to axon regeneration. Eye 31:206–208
Ferhat L, Au Louis NC, Jorquera I et al (1996) Transient increase of tenascin-C in immature hippocampus: astroglial and neuronal expression. J Neurocytol 25:53–66
Ferrer-Ferrer M, Dityatev A (2018) Shaping synapses by the neural extracellular matrix. Front Neuroanat 12:40
Fidler PS, Schuette K, Asher RA et al (1999) Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci 19:8778–8788
Filous AR, Schwab JM (2018) Determinants of axon growth, plasticity, and regeneration in the context of spinal cord injury. Am J Pathol 188:53–62
Filous AR, Tran A, Howell CJ et al (2014) Entrapment via synaptic-like connections between NG2 proteoglycan + cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci 34:16369–16384
Foscarin S, Ponchione D, Pajaj E et al (2011) Experience-dependent plasticity and modulation of growth regulatory molecules at central synapses. PLoS One 6:166–172
Freitag S, Schachner M, Morellini F (2003) Behavioral alterations in mice deficient for the extracellular matrix glycoprotein tenascin-R. Behav Brain Res 145:189–207
Frischknecht R, Seidenbecher CI (2012) Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol 44:1051–1054
Frischknecht R, Heine M, Perrais D et al (2009) Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12:897
Fujita Y, Yamashita T (2014) Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci 8:338
Fukushi J, Makagiansar IT, Stallcup WB (2004) NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and α3β1 integrin. Mol Biol Cell 15:3580–3590
Galtrey CM, Fawcett JW (2007) The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54:1–18
Galtrey CM, Kwok JCF, Carulli D et al (2008) Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci 27:1373–1390
Geissler M, Gottschling C, Aguado A et al (2013) Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J Neurosci 33:7742–7755
Geoffroy CG, Zheng B (2014) Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol 27:31–38
Giamanco KA, Matthews RT (2012) Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience 218:367–384
Giblin SP, Midwood KS (2015) Tenascin-C: form versus function. Cell Adh Migr 9:48–82
Giuffrida A, Scarpa S, Birarelli P, Modesti A (2004) The interaction of tenascin-C with fibronectin modulates the migration and specific metalloprotease activity in human mesothelioma cell lines of different histotype. Int J Oncol 25:745–750
Gokce O, Südhof TC (2013) Membrane-tethered monomeric neurexin LNS-domain triggers synapse formation. J Neurosci 33:14617–14628
Gottschling C, Wegrzyn D, Denecke B, Faissner A (2019) Elimination of the four extracellular matrix molecules tenascin-C, tenascin-R, brevican and neurocan alters the ratio of excitatory and inhibitory synapses. Sci Rep 9:1–17
Guntinas-Lichius O, Angelov DN, Morellini F et al (2005) Opposite impacts of tenascin-C and tenascin-R deficiency in mice on the functional outcome of facial nerve repair. Eur J Neurosci 22:2171–2179
Gurevicius K, Gureviciene I, Valjakka A et al (2004) Enhanced cortical and hippocampal neuronal excitability in mice deficient in the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci 25:515–523
Gurevicius K, Kuang F, Stoenica L et al (2009) Genetic ablation of tenascin-C expression leads to abnormal hippocampal CA1 structure and electrical activity in vivo. Hippocampus 19:1232–1246
Gutowski NJ, Newcombe J, Cuzner ML (1999) Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathol Appl Neurobiol 25:207–214
Happel MFK, Niekisch H, Rivera LLC et al (2014) Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci 111:2800–2805
Härtig W, Brauer K (1992) Agglutinin-labelled nets surround parvalbumin-containing neurons. NeuroReport 3:872
Hascall VC, Heinegård D (1974) Aggregation of cartilage proteoglycans II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem 249:4242–4249
Hausen D, Brückner G, Drlicek M et al (1996) Pyramidal cells ensheathed by perineuronal nets in human motor and somatosensory cortex. NeuroReport 7:1725–1729
Hayashi N, Mizusaki MJ, Kamei K et al (2005) Chondroitin sulfate proteoglycan phosphacan associates with parallel fibers and modulates axonal extension and fasciculation of cerebellar granule cells. Mol Cell Neurosci 30:364–377
Hayashi MK, Nishioka T, Shimizu H et al (2019) Hyaluronan synthesis supports glutamate transporter activity. J Neurochem 150:249–263
Heikkinen A, Pihlajaniemi T, Faissner A, Yuzaki M (2014) Neural ECM and synaptogenesis. In: Progress in brain research, vol 16. Elsevier, Amsterdam, pp 29–51
Heine M, Groc L, Frischknecht R et al (2008) Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320:201–205
Hillen AEJ, Burbach JPH, Hol EM (2018) Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog Neurobiol 165:66–86
Hirakawa S, Oohashi T, Su W-D et al (2000) The brain link protein-1 (BRAL1): cDNA cloning, genomic structure, and characterization as a novel link protein expressed in adult brain. Biochem Biophys Res Commun 276:982–989
Hossain-Ibrahim MK, Rezajooi K, Stallcup WB et al (2007) Analysis of axonal regeneration in the central and peripheral nervous systems of the NG2-deficient mouse. BMC Neurosci 8:80
Howell MD, Bailey LA, Cozart MA et al (2015) Hippocampal administration of chondroitinase ABC increases plaque-adjacent synaptic marker and diminishes amyloid burden in aged APPswe/PS1dE9 mice. Acta Neuropathol Commun 3:54
Huang W, Chiquet-Ehrismann R, Moyano JV et al (2001) Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res 61:8586–8594
Huang C, Sakry D, Menzel L et al (2016) Lack of NG 2 exacerbates neurological outcome and modulates glial responses after traumatic brain injury. Glia 64:507–523
Irintchev A, Rollenhagen A, Troncoso E et al (2005) Structural and functional aberrations in the cerebral cortex of tenascin-C deficient mice. Cereb Cortex 15:950–962
Irvine SF, Kwok JCF (2018) Perineuronal nets in spinal motoneurones: chondroitin sulphate proteoglycan around alpha motoneurones. Int J Mol Sci 19:1172
Itano N, Sawai T, Yoshida M et al (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274:25085–25092
Jakovcevski I, Miljkovic D, Schachner M, Andjus PR (2013) Tenascins and inflammation in disorders of the nervous system. Amino Acids 44:1115–1127
Jayakumar AR, Apeksha A, Norenberg MD (2017) Role of matricellular proteins in disorders of the central nervous system. Neurochem Res 42:858–875
Joester A, Faissner A (2001) The structure and function of tenascins in the nervous system. Matrix Biol 20:13–22
Jones EV, Bouvier DS (2014) Astrocyte-secreted matricellular proteins in CNS remodelling during development and disease. Neural Plast. https://doi.org/10.1155/2014/321209
Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH (2002) NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci 22:2792–2803
Joo NE, Miao D, Bermúdez M et al (2014) Shedding of NG2 by MMP-13 attenuates anoikis. DNA Cell Biol 33:854–862
Kochlamazashvili G, Henneberger C, Bukalo O et al (2010) The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2 + channels. Neuron 67:116–128
Korotchenko S, Zanacchi FC, Diaspro A, Dityatev A (2014) Zooming in on the (Peri) synaptic extracellular matrix. In: Nanoscale imaging of synapses, vol 2014. Springer, Berlin, pp 187–203
Krishnaswamy VR, Benbenishty A, Blinder P, Sagi I (2019) Demystifying the extracellular matrix and its proteolytic remodeling in the brain: structural and functional insights. Cell Mol Life Sci 76:3229–3248
Kucharova K, Stallcup WB (2018) Dissecting the multifactorial nature of demyelinating disease. Neural Regen Res 13:628
Kucukdereli H, Allen NJ, Lee AT et al (2011) Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci 108:E440–E449
Kula B, Chen T, Kukley M (2019) Glutamatergic signaling between neurons and oligodendrocyte lineage cells: is it synaptic or non-synaptic? Glia 67:2071–2091
Kwok JCF, Carulli D, Fawcett JW (2010) In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J Neurochem 114:1447–1459
Kwok JCF, Dick G, Wang D, Fawcett JW (2011) Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71:1073–1089
Lander C, Zhang H, Hockfield S (1998) Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J Neurosci 18:174–183
Lang BT, Cregg JM, DePaul MA et al (2015) Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518:404–408
Lasek AW, Chen H, Chen W-Y (2018) Releasing addiction memories trapped in perineuronal nets. Trends Genet 34:197–208
Lee S, Zhang W, Ravi M et al (2013) Atypical protein kinase C and Par3 are required for proteoglycan-induced axon growth inhibition. J Neurosci 33:2541–2554
Lendvai D, Morawski M, Négyessy L et al (2013) Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer’s disease. Acta Neuropathol 125:215–229
Lensjø KK, Christensen AC, Tennøe S et al (2017) Differential expression and cell-type specificity of perineuronal nets in hippocampus, medial entorhinal cortex, and visual cortex examined in the rat and mouse. eneuro. https://doi.org/10.1523/ENEURO.0379-16.2017
Li Y, Li Z-X, Jin T et al (2017) Tau pathology promotes the reorganization of the extracellular matrix and inhibits the formation of perineuronal nets by regulating the expression and the distribution of hyaluronic acid synthases. J Alzheimer’s Dis 57:395–409
Liu H, Shubayev VI (2011) Matrix metalloproteinase-9 controls proliferation of NG2 + progenitor cells immediately after spinal cord injury. Exp Neurol 231:236–246
Liu J, Gao H, Wang X (2015) The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res 10:1892
Lundell A, Olin AI, Mörgelin M et al (2004) Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure 12:1495–1506
Maleski M, Hockfield S (1997) Glial cells assemble hyaluronan-based pericellular matrices in vitro. Glia 20:193–202
Marks MS, Chi-Rosso G, Toole BP (1990) Hyaluronate-binding proteins of murine brain. J Neurochem 54:171–180
McTigue DM, Tripathi R, Wei P (2006) NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol 65:406–420
Milev P, Chiba A, Häring M et al (1998) High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-ζ/β with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J Biol Chem 273:6998–7005
Miller GM, Hsieh-Wilson LC (2015) Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans. Exp Neurol 274:115–125
Mitlöhner J, Kaushik R, Niekisch H et al (2020) Dopamine receptor activation modulates the integrity of the perisynaptic extracellular matrix at excitatory synapses. Cells 9:260
Miyata S, Kitagawa H (2017) Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta 1861:2420–2434
Miyata S, Nishimura Y, Hayashi N, Oohira A (2005) Construction of perineuronal net-like structure by cortical neurons in culture. Neuroscience 136:95–104
Morawski M, Brückner G, Jäger C et al (2012) Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer’s disease neuropathology. Brain Pathol 22:547–561
Morawski M, Dityatev A, Hartlage-Rübsamen M et al (2014) Tenascin-R promotes assembly of the extracellular matrix of perineuronal nets via clustering of aggrecan. Philos Trans R Soc B Biol Sci 369:20140046
Morellini F, Sivukhina E, Stoenica L et al (2010) Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex 20:2712–2727
Morgenstern DA, Asher RA, Naidu M et al (2003) Expression and glycanation of the NG2 proteoglycan in developing, adult, and damaged peripheral nerve. Mol Cell Neurosci 24:787–802
Mufson EJ, Mahady L, Waters D et al (2015) Hippocampal plasticity during the progression of Alzheimer’s disease. Neuroscience 309:51–67
Muramatsu T (2002) Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 132:359–371
Naba A, Hoersch S, Hynes RO (2012) Towards definition of an ECM parts list: an advance on GO categories. Matrix Biol J Int Soc Matrix Biol 31:371
Naba A, Clauser KR, Ding H et al (2016) The extracellular matrix: tools and insights for the “omics” era. Matrix Biol 49:10–24
Nakamura A, Morise J, Yabuno-Nakagawa K et al (2019) Site-specific HNK-1 epitope on alternatively spliced fibronectin type-III repeats in tenascin-C promotes neurite outgrowth of hippocampal neurons through contactin-1. PLoS ONE 14:122–128
Nakic M, Manahan-Vaughan D, Reymann KG, Schachner M (1998) Long-term potentiation in vivo increases rat hippocampal tenascin-C expression. J Neurobiol 37:393–404
Nayak T, Trotter J, Sakry D (2018) The intracellular cleavage product of the NG2 proteoglycan modulates translation and cell-cycle kinetics via effects on mTORC1/FMRP signaling. Front Cell Neurosci 12:231
Neame PJ, Christner JE, Baker JR (1986) The primary structure of link protein from rat chondrosarcoma proteoglycan aggregate. J Biol Chem 261:3519–3535
Nikonenko I, Jourdain P, Muller D (2003) Presynaptic remodeling contributes to activity-dependent synaptogenesis. J Neurosci 23:8498–8505
Niquet J, Jorquera I, Faissner A et al (1995) Gliosis and axonal sprouting in the hippocampus of epileptic rats are associated with an increase of tenascin-C immunoreactivity. J Neurocytol 24:611–624
Nishihara T, Remacle AG, Angert M et al (2015) Matrix metalloproteinase-14 both sheds cell surface neuronal glial antigen 2 (NG2) proteoglycan on macrophages and governs the response to peripheral nerve injury. J Biol Chem 290:3693–3707
Nishiyama A, Dahlin KJ, Prince JT et al (1991) The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol 114:359–371
Nishiyama A, Lin X-H, Stallcup WB (1995) Generation of truncated forms of the NG2 proteoglycan by cell surface proteolysis. Mol Biol Cell 6:1819–1832
Nishiyama A, Komitova M, Suzuki R, Zhu X (2009) Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10:9–22
Ohtake Y, Li S (2015) Molecular mechanisms of scar-sourced axon growth inhibitors. Brain Res 1619:22–35
Oohashi T, Hirakawa S, Bekku Y et al (2002) Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Mol Cell Neurosci 19:43–57
Oohashi T, Edamatsu M, Bekku Y, Carulli D (2015) The hyaluronan and proteoglycan link proteins: organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol 274:134–144
Orlando C, Ster J, Gerber U et al (2012) Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci 32:18009–18017
Passlick S, Trotter J, Seifert G et al (2016) The NG2 Protein Is Not Required for Glutamatergic Neuron–NG2 Cell Synaptic Signaling. Cereb Cortex 26:51–57
Paukert M, Bergles DE (2006) Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol 16:515–521
Pesheva P, Gennarini G, Goridis C, Schachner M (1993) The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron 10:69–82
Pizzorusso T, Medini P, Berardi N et al (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251
Probstmeier R, Braunewell K-H, Pesheva P (2000) Involvement of chondroitin sulfates on brain-derived tenascin-R in carbohydrate-dependent interactions with fibronectin and tenascin-C. Brain Res 863:42–51
Pyka M, Wetzel C, Aguado A et al (2011) Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur J Neurosci 33:2187–2202
Ramón y Cajal S (1928) Degeneration and regeneration of the nervous system
Reinhard J, Roll L, Faissner A (2017) Tenascins in retinal and optic nerve neurodegeneration. Front Integr Neurosci 11:30
Rezajooi K, Pavlides M, Winterbottom J et al (2004) NG2 proteoglycan expression in the peripheral nervous system: upregulation following injury and comparison with CNS lesions. Mol Cell Neurosci 25:572–584
Richter RP, Baranova NS, Day AJ, Kwok JCF (2018) Glycosaminoglycans in extracellular matrix organisation: are concepts from soft matter physics key to understanding the formation of perineuronal nets? Curr Opin Struct Biol 50:65–74
Roll L, Faissner A (2019) Tenascins in CNS lesions. In: Seminars in cell & developmental biology, vol 26. Elsevier, Amsterdam, pp 118–124
Roszkowska M, Skupien A, Wójtowicz T et al (2016) CD44: a novel synaptic cell adhesion molecule regulating structural and functional plasticity of dendritic spines. Mol Biol Cell 27:4055–4066
Rudenko G, Nguyen T, Chelliah Y et al (1999) The structure of the ligand-binding domain of neurexin Ib: regulation of LNS domain function by alternative splicing. Cell 99:93–102
Saghatelyan AK, Gorissen S, Albert M et al (2000) The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur J Neurosci 12:3331–3342
Saghatelyan AK, Dityatev A, Schmidt S et al (2001) Reduced perisomatic inhibition, increased excitatory transmission, and impaired long-term potentiation in mice deficient for the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci 17:226–240
Saghatelyan AK, Snapyan M, Gorissen S et al (2003) Recognition molecule associated carbohydrate inhibits postsynaptic GABAB receptors: a mechanism for homeostatic regulation of GABA release in perisomatic synapses. Mol Cell Neurosci 24:271–282
Sakry D, Trotter J (2016) The role of the NG2 proteoglycan in OPC and CNS network function. Brain Res 1638:161–166
Sakry D, Karram K, Trotter J (2011) Synapses between NG2 glia and neurons. J Anat 219:2–7
Sakry D, Neitz A, Singh J et al (2014) Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol 12:e1001993
Schäfer MKE, Tegeder I (2018) NG2/CSPG4 and progranulin in the posttraumatic glial scar. Matrix Biol 68:571–588
Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384
Schultz N, Nielsen HM, Minthon L, Wennström M (2014) Involvement of matrix metalloproteinase-9 in amyloid-β 1–42–induced shedding of the pericyte proteoglycan NG2. J Neuropathol Exp Neurol 73:684–692
Seidenbecher CI, Smalla K, Fischer N et al (2002) Brevican isoforms associate with neural membranes. J Neurochem 83:738–746
Šekeljić V, Andjus PR (2012) Tenascin-C and its functions in neuronal plasticity. Int J Biochem Cell Biol 44:825–829
Senkov O, Andjus P, Radenovic L, et al (2014) Neural ECM molecules in synaptic plasticity, learning, and memory. In: Progress in brain research, vol 26. Elsevier, pp 53–80
Shen Y, Tenney AP, Busch SA et al (2009) PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592–596
Siri A, Knäuper V, Veirana N et al (1995) Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem 270:8650–8654
Smith GM, Hale JH (1997) Macrophage/microglia regulation of astrocytic tenascin: synergistic action of transforming growth factor-β and basic fibroblast growth factor. J Neurosci 17:9624–9633
Smith PD, Coulson-Thomas VJ, Foscarin S et al (2015) “GAG-ing with the neuron”: the role of glycosaminoglycan patterning in the central nervous system. Exp Neurol 274:100–114
Sonntag M, Blosa M, Schmidt S et al (2015) Perineuronal nets in the auditory system. Hear Res 329:21–32
Sonntag M, Blosa M, Schmidt S et al (2018) Synaptic coupling of inner ear sensory cells is controlled by brevican-based extracellular matrix baskets resembling perineuronal nets. BMC Biol 16:99
Sorg BA, Berretta S, Blacktop JM et al (2016) Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci 36:11459–11468
Spicer AP, Joo A, Bowling RA (2003) A hyaluronan binding link protein gene family whose members are physically linked adjacent to chrondroitin sulfate proteoglycan core protein genes the missing links. J Biol Chem 278:21083–21091
Srivastava T, Sherman LS, Back SA (2020) Dysregulation of hyaluronan homeostasis during white matter injury. Neurochem Res 45:672–683
Stallcup WB, Huang F-J (2008) A role for the NG2 proteoglycan in glioma progression. Cell Adh Migr 2:192–201
Stamenkovic V, Stamenkovic S, Jaworski T et al (2017) The extracellular matrix glycoprotein tenascin-C and matrix metalloproteinases modify cerebellar structural plasticity by exposure to an enriched environment. Brain Struct Funct 222:393–415
Stegmüller J, Werner H, Nave K-A, Trotter J (2003) The proteoglycan NG2 is complexed with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells implications for glial-neuronal signaling. J Biol Chem 278:3590–3598
Steindler DA, Cooper NGF, Faissner A, Schachner M (1989) Boundaries defined by adhesion molecules during development of the cerebral cortex: the J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Dev Biol 131:243–260
Strekalova T, Sun M, Sibbe M et al (2002) Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol Cell Neurosci 21:173–187
Sugahara K, Mikami T, Uyama T et al (2003) Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol 13:612–620
Sugiura N, Shioiri T, Chiba M et al (2012) Construction of a chondroitin sulfate library with defined structures and analysis of molecular interactions. J Biol Chem 287:43390–43400
Suttkus A, Rohn S, Weigel S et al (2014) Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis 5:e1119–e1119
Swindle CS, Tran KT, Johnson TD et al (2001) Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol 154:459–468
Tamburini E, Dallatomasina A, Quartararo J et al (2019) Structural deciphering of the NG2/CSPG4 proteoglycan multifunctionality. FASEB J 33:3112–3128
Tan AM, Zhang W, Levine JM (2005) NG2: a component of the glial scar that inhibits axon growth. J Anat 207:717–725
Tan AM, Colletti M, Rorai AT et al (2006) Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci 26:4729–4739
Tan CL, Andrews MR, Kwok JCF et al (2012) Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J Neurosci 32:7325–7335
Testa D, Prochiantz A, Di Nardo AA (2019) Perineuronal nets in brain physiology and disease. In: Seminars in cell & developmental biology, vol 16. Elsevier, Amsterdam, pp 125–135
Tillet E, Ruggiero F, Nishiyama A, Stallcup WB (1997) The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem 272:10769–10776
Tucker RP, Chiquet-Ehrismann R (2015) Tenascin-C: its functions as an integrin ligand. Int J Biochem Cell Biol 65:165–168
Vargová L, Syková E (2014) Astrocytes and extracellular matrix in extrasynaptic volume transmission. Philos Trans R Soc B Biol Sci 369:20130608
Vedunova M, Sakharnova T, Mitroshina E et al (2013) Seizure-like activity in hyaluronidase-treated dissociated hippocampal cultures. Front Cell Neurosci 7:149
Vo T, Carulli D, Ehlert EME et al (2013) The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol Cell Neurosci 56:186–200
Vollmer G, Tan MI, Wünsche W, Frank K (1997) Expression of tenascin-C by human endometrial adenocarcinoma and stroma cells: heterogeneity of splice variants and induction by TGF-b. Biochem Cell Biol 75:759–769
Vyavahare N, Jones PL, Tallapragada S, Levy RJ (2000) Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol 157:885–893
Walker CD, Risher WC, Risher M-L (2020) Regulation of synaptic development by astrocyte signaling factors and their emerging roles in substance abuse. Cells 9:297
Wang D, Ichiyama RM, Zhao R et al (2011) Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31:9332–9344
Weber P, Bartsch U, Rasband MN et al (1999) Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci 19:4245–4262
Wegner F, Härtig W, Bringmann A et al (2003) Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABAA receptor α1 subunit form a unique entity in rat cerebral cortex. Exp Neurol 184:705–714
Wen Y, Makagiansar IT, Fukushi J et al (2006) Molecular basis of interaction between NG2 proteoglycan and galectin-3. J Cell Biochem 98:115–127
Wennström M, Janelidze S, Bay-Richter C et al (2014) Pro-inflammatory cytokines reduce the proliferation of NG2 cells and increase shedding of NG2 in vivo and in vitro. PLoS ONE 9:18–25
Wiese S, Karus M, Faissner A (2012) Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol 3:120
Wlodarczyk J, Mukhina I, Kaczmarek L, Dityatev A (2011) Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev Neurobiol 71:1040–1053
Wu X, Xu X (2016) RhoA/Rho kinase in spinal cord injury. Neural Regen Res 11:23
Xiao Z, Revest J, Laeng P et al (1998) Defasciculation of neurites is mediated by tenascin-R and its neuronal receptor F3/11. J Neurosci Res 52:390–404
Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y (1994) Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem 269:10119–10126
Yamamoto S, Oka S, Inoue M et al (2002) Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J Biol Chem 277:27227–27231
Yang H, Xiao Z, Becker B et al (1999) Role for myelin-associated glycoprotein as a functional tenascin-R receptor. J Neurosci Res 55:687–701
Yang Z, Suzuki R, Daniels SB et al (2006) NG2 glial cells provide a favorable substrate for growing axons. J Neurosci 26:3829–3839
Yasuhara O, Akiyama H, McGeer EG, McGeer PL (1994) Immunohistochemical localization of hyaluronic acid in rat and human brain. Brain Res 635:269–282
You J, Hong S-Q, Zhang M-Y et al (2012) Passive immunization with tenascin-R (TN-R) polyclonal antibody promotes axonal regeneration and functional recovery after spinal cord injury in rats. Neurosci Lett 525:129–134
You W-K, Yotsumoto F, Sakimura K et al (2014) NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis 17:61–76
Yu P, Pearson CS, Geller HM (2018) Flexible roles for proteoglycan sulfation and receptor signaling. Trends Neurosci 41:47–61
Funding
This study was supported by Grant 19-015-00018a (Y.A. Chelyshev) from the Russian Foundation for Basic Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chelyshev, Y.A., Kabdesh, I.M. & Mukhamedshina, Y.O. Extracellular Matrix in Neural Plasticity and Regeneration. Cell Mol Neurobiol 42, 647–664 (2022). https://doi.org/10.1007/s10571-020-00986-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00986-0