Abstract
In the present work, amine-functionalized nanodiamonds (NDs) have been encapsulated in liposomes and studied in order to observe the modification of their photoluminescence properties. NDs were functionalized with aromatic amines such as 1-aminopyrene and 2-aminofluorene, and the aliphatic amine 1-octadecylamine. Morphology, structural and optical properties of NDs and amine-modified NDs were analyzed by transmission electron microscopy, atomic force microscopy, scanning electron microscopy, and photoluminescence. The amine-functionalized NDs were successfully encapsulated in lecithin liposomes prepared by the green and conventional methods. The obtained results show significant changes in photoluminescent properties of functionalized NDs, and were more potentialized after liposome encapsulation. Our findings could be applied in the development of new kinds of water-dispersible fluorescent hybrids, liposome-NDs, with the capability of drug encapsulation for use in diagnostics and therapy (theragnostic liposomes). All-optical sensors with possibilities for tailoring their response for other biomedical applications can be also contemplated.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The combination of optical properties such as Raman gain, strong linear and nonlinear refractive index, wide bandgap, and high stability of emission (Laube et al 2019), makes nanodiamonds (NDs) a very promising material for research and technological applications. Besides, the large surface area derived by surface modification or doping, promotes a reduced cytotoxicity in NDs with a crucial potential in sensing and instrumentation fields. Nanoelectromechanical systems (NEMS), magnetic and electric field sensors, integrated Raman lasers, frequency combs, bio-medicine probes, bio-sensors, bio-imaging by cell labeling/monitoring, and drug delivery systems are some attractive examples for NDs applications (Loncar and Faraon 2013, Kumar et al 2013, Xiao et al 2015, Lake and Bouchard 2017, Pichot et al 2017, Tinwala and Wairkar 2019).
Diverse biomedical functions can be better explored or handled by using dispersible NDs in stable aqueous solutions. Therefore, the application of liposomes to encapsulate NDs seems to be a suitable area of research. Above all, it is well-known that liposomes allow encapsulation of drugs or other substances. The liposomes consist of phospholipid bilayers that self-close to form spherical nanoparticles. Due to the presence of hydrophilic internal volume and phospholipid bilayers, amphiphilic substances can be delivered by liposomes. Recent developments of theragnostic medicine combine therapy and diagnostic in the same vehicle for developing optical methods of detection (Pham et al 2017, Seleci et al 2017, and references therein). The theragnostic applications of liposomes can be particularly useful for drug delivery associated with cancer and other diseases therapy (Turcheniuk and Mochalin 2017, Morales-Zavala et al 2018, Patra et al 2018). The new theragnostic medicine permits monitoring in vitro and in vivo drug pharmacokinetics and pharmacologic efficiency studies (Jia et al 2019). The theragnostic liposomes with the NDs applications may improve their optical properties, specially for applications related to nano biosensors, and optoelectronic devices. Another advantage of the liposomes concerns the potential conjugation of their surface with other molecules as target agents (peptides, antibodies, cations), resulting in selectivity, or their targeted delivery into cells, systems, and organs of the body; and stability in biologic media.
Therefore, the hypothesis of this study is that the NDs with different amines on their surfaces, and liposome-encapsulated, can give modification of the optical properties up to enhanced luminescence. And this in turn, can allow luminescence detection of these hybrids for their application in theragnostic liposomes and biosensors.
Multiple applications of NDs are based on the chemical and structural properties of NDs. The diamond core is composed of sp3, and the amorphous shell by sp2 hybridized carbon atoms (Zhang et al 2018). The dense packing of sp3 in the core of NDs is responsible for their chemical and photostability (Giulia 2010, Hemelaar et al 2017, Laube et al 2019). The sp2 hybridization in NDs surface leads to a versatile chemical nature and makes this nanomaterial suitable for chemical functionalization (Pichot et al 2017).
Therefore, in this work, we analyzed the influence of functionalization and encapsulation of NDs in their optical properties. Herein is identified for the first time the signature that corresponds to optical emission of this type of hybrids based on liposomes with pristine and functionalized NDs encapsulates. To our knowledge, there are no reports where the encapsulation of NDs is presented, nor of their amino-functionalized versions in liposomes and their PL properties for applications in theragnostic liposomes and the possibility for revealing them by this method. This also includes fluorescent microscopy, which is used for imaging and emission in areas of biology and medicine. Thus, the role of liposome encapsulation of amine-functionalized NDs seems to be a key aspect for developing tailored nanoprobes using fluorescent NDs. And, as already mentioned, the liposome encapsulation of NDs may offer their water dispersibility for biomedical applications as biomarkers in theragnostic liposomes.
Different synthesis methods of NDs have been reported, but only the main detonation techniques that correspond to laser ablation, and high-energy ball milling of high-pressure with high-temperature are being used commercially (Fang et al 2013, Guillou et al 2007) . The pristine NDs possess a large bandgap of 5.5 eV, and like all semiconductor quantum dots, they possess high photostability and bright multicolor fluorescence; however, their core emission may increase up to 8.8–9.1eV by decreasing their particle size (Mochalin et al 2012).
The optical properties of doped NDs are highly dependent on their synthesis methods and on the nature of the dopant (Fokin and Schreiner 2009). The color centers are developed due to a compression in NDs structure by an impurity atom (N, Si, B or Cr), a vacant lattice site, or a trapped electron, and due to that, the energy gap can be decreased to the similar value of bulk diamonds (Dolenko et al 2012). The dominant impurity on NDs is nitrogen, forming vacancies adjacent to the substitutional nitrogen atoms color centers (NV) of 2p origin (Fokin and Schreiner 2009, Kovalenko et al 2012). And, because of this, HOMO and LUMO of nitrogen p-electrons by the addition of new levels may reduce the band gap in NDs. This in turn, becomes one of the most important properties of NV in NDs: an easily detectable luminescence in visible spectra, highly desired for biomedical applications with no photo-bleaching or blinking (Loncar and Faraon 2013, Lake and Bouchard 2017). However, frequency-selective photonic control is still a challenge due to the NV broadband fluorescence emission compared to other quantum emitters at room temperature. Thus, the H3 defect center (N-V-N) in 35 nm size diamonds has green emission (at 531 nm), and NDs functionalized with octadecylamine (ODA) present additional blue emission (at 480 nm) to the set of red and green colors of pristine NDs (Mochalin and Gogotsi 2009).
Also, the pristine NDs surface containing disordered carbon, and small graphitic nanoclusters, may add excitation levels in the NDs due to structural inhomogeneity, defects, and mixture (Mona et al 2012, Treussart and Vlasov 2017). The amorphous core´s fluorescence could be enhanced due to the surface functionalization, the surrounding medium, and pH (Mona et al 2012, Mochalin et al 2012). The theoretical simulations of band gaps have been performed for NDs particles of sizes from 1 to 3.3 nm, and it has been identified that the quantum confinement effects strongly dependent on particle size, morphology, shape, and the incorporation of heteroatom functionalities (Drummond et al 2005, Barnard 2009, Fokin and Schreiner 2009). The broadband between 400–600 nm is also observed for almost all nanocrystalline diamonds with sp2 phase between and on the nanocrystal surfaces, where the band at 430 nm (in the blue spectral region) corresponds to volume defects, recently assigned to –OH bonds; and at around 520 nm to –COOH and C–O–C groups (Xiao et al 2015, Stehlik et al 2016, and references therein). The NDs surface often has charge traps that involve non-radiative recombination pathways. Upon introduction of the coating of the molecule, such traps are suppressed, and several non-radiative recombination channels are eliminated. However, a priori measurements indicate that the quantum efficiency of a single NV center in a NDs is not unity, and it can be significantly improved by using a coating with organic molecules, resulting in brighter fluorescent NDs (Bray et al 2015, and references therein). Changes in shape and spectral positions of the NDs PL band have been observed and found to depend on the excitation wavelength. With this motivation, we employed a standard 355 nm excitation wavelength for PL measurements in this work; this wavelength has not been used in comparative publications. Emission peaks of high energy, related to this double modification of NDs surfaces by the assistance of amine-functionalization, and liposome encapsulation were analyzed.
Amplification in PL phenomena in encapsulated NDs can be associated with surface energy traps elimination and transfer between proteins and surface nanostructures, particularly the graphite-like nanoclusters. These considerations are important for biological and medical applications of NDs used as a biocompatible label (Perevedentseva et al 2011).
As we mentioned before, the organic fluorophores may promote quenching by resonance energy transfer to the non-fluorescent group (Lakowicz 1999). Thus, fluorene-based conjugated compounds have emerged for the use as light-emitting diodes due to their high PL and electroluminescence quantum efficiencies, easy film-forming, thermal stability, good solubility, and ready color-tuning through the incorporation of low-band-gap co-monomers. Several approaches based on local electromagnetic environment modifications have been explored to tune the energy of emitted photons. The modifications in the spectral shifts and intensity increase in PL signals could be induced, enhanced, and controlled by attaching different organic molecules, halogens, and surrounding media variations (Mochalin et al 2012, Mona et al 2012, Hasanzadeh et al 2019).
In addition, other poly-nuclear aromatic hydrocarbons may form excimers (the dimer components in the excited states, or their complexes) and upgrade non-linear optical response (Rourke et al 2011, Du et al 2015). The hyposochromic shift of energy (up to higher energies) in the PL spectra of NDs could be obtained from removing the amorphous carbon and surrounding the crystalline NDs due to their interaction with 1-undecene (Kompan et al 1997).
Based on our previous studies on other biomarkers encapsulated in liposomes, we know that the liposome encapsulation of semiconductor quantum dots of CdSe and CdSe/ZnS may produce additional surface tension, and subsequently, luminescence properties enhancement (Douda et al 2018 and 2019a). Recently we reported studies on optical properties modification of graphene oxide chemically functionalized by amine molecules (Douda et al 2019b).
It is worth mentioning that in the references about the NDs PL, the excitation only up to 405 nm and lower have been employed (Chung et al 2007, Mona et al 2012, Turcheniuk and Mochalin 2017, Afandi et al 2018). Su and coworkers (2016), report transient fluorescence properties of the NDs under excitation at 280 nm wavelength of PL decay curves, and only aliphatic amine 1-octadecylamine (ODA) and 1-undecene functionalized NDs (Mochalin and Gogotsi 2009, Astuti et al 2014), respectively.
In the present work, we studied the optical properties of the pristine and amine-functionalized NDs before and after encapsulation in liposomes; a 355 nm laser beamline was used to excite the NDs samples.
NDs were functionalized with two aromatic amines: 1-aminopyrene (AP) and 2-aminofluorene (AF), and one aliphatic amine, ODA by method for chemical functionalization widely used by Basiuk and collaborators to functionalize carbon nanomaterials (Basiuk et al 2012 , 2013, and references therein).
After that, the NDs pristine and amine-functionalized were encapsulated in liposomes, prepared from soybean lecithin by two different methods. The optical properties of the obtained samples were systematically studied by PL measurements. The PL spectra of all functionalized materials showed significant changes dependent on the amine structure attached to NDs surfaces, and they were more pronounced after the liposome encapsulation. We highlight that no change between PL results for both encapsulation methods (conventional and green) were observed, which suggest that any type of liposomes can give the effect of increasing emission energy in NDs PL. We state that PL properties of NDs can be tuned through chemical functionalization of NDs with amine molecules and subsequent liposome-encapsulation, which opens the door to further development of new theragnostic liposomes, light-emitting devices, and nano biosensors based on such systems.
2. Materials and methods
NDs samples featuring a 6 nm average size and functionalized with AP, AF, and ODA by method widely reported (Alzate-Carvajal et al 2016), and amino-functionalized ND samples were obtained: ND-AP, ND-AF and ND-ODA, respectively.
The liposomes encapsulation of NDs was performed by a 'green' method (without organic solvent application), reported before ( Douda et al 2019a ) from commercial soybean lecithin (liquid and dried). Briefly, the dispersion of soybean lecithin (120 g l−1 g) in an aqueous solution of 10−5 M NaCl was swollen during 24 h. For each sample, 60 μg of the lecithin previously prepared, a lecithin solution was taken, mixed with the NDs samples and (phosphate buffer saline) PBS to obtain a total amount of 500 μl. The liposome-encapsulated NDs samples obtained by the 'green' method were: LND, LND-ODA, LND-AP, LND-AF.
The sonication process was carried out with ultrasonic equipment (Sonics and Materials, VCX-130) at 20 kHz equipped with a Titanium Probe (6 mm in diameter) for 20 min. Preselected cycles of 5-s alternate on-off program with a total treatment time of 5 min were applied. The NDs and their functionalized samples were added to the dried phospholipids bilayer before rehydration by PBS (pH = 7.4). Cooling up to −20 °C was applied after all sonication processes and the samples were delivered to ambient temperatures before further sonication. The process was repeated 3 times for all samples prepared by triplicate.
The conventional method of liposomes preparation is known as the lipid bilayer rehydration method (Amselem et al 1990). A solution of 100mg of soybean lecithin powder in 10 ml of chloroform/methanol (9:1, v:v) was prepared, 400 μl of this solution was taken in a round bottom tube to which one of the NDs was added. A vacuum atmosphere was employed until the organic solvents were completely evaporated. The thin lipid bilayer obtained was left vacuumed overnight to remove traces of solvent. The resulting thin film was hydrated with 2 ml of PBS (pH = 7.4), and sonicated for 30 min with a power output of 65 W. The solution obtained was extruded in a mini Extruder Avanti Polar Lipid with a 0.1 μm polycarbonate membrane 11 times. Subsequently, it was centrifuged at 38 000 RPM for 60 min in the ultracentrifuge Beckman Coultier 90L, and the obtained pellet was resuspended in PBS. The solution was stored in refrigeration for further analysis. The liposomes prepared by conventional methods for NDs encapsulation were: pure liposome, L*ND, L*ND-AP, L*ND-AF.
For the morphology characterization, SEM observations of sample powder were performed in a JEOL JSM-6510LV at low voltage (5 kV), while for TEM images, samples diluted in ethanol were deposited as droplets in Lacey copper grids and were observed in a Tecnai F30 high-resolution analytical transmission electron microscope (300 kV). The atomic force microscopy (AFM) measurements were made by using an AFM multi-mode system in taping mode.
The PL spectra were measured by exciting the samples with the third harmonic of a Nd:YAG laser (λ = 355 nm, 25 ps ) at 300 K using a UV-VIS spectrometer (USB2000 + Ocean Optics) connected to an optical fiber. For most of the samples studied in this work, PL emissions were visible to the naked eye. This PL condition made the implementation of our experimental setup easy in different directions of the emission. The PL light coming from the samples was collected with a convex lens of 5 cm in diameter, in order to acquire the PL light in our experimental setup. The optical fiber was carefully placed in the convex lens focus and coupled to the acceptance angle of the fiber. The PL measurements were performed on liquid samples for 'green' liposomes. For the conventional method of liposome preparation, the suspensions of pristine and functionalized NDs in PBS or ethanol were dried in the form of droplets on Si substrate. For liquid samples, the PL emissions were detected in a right-angle configuration to avoid the reflected laser signal. In the case of deposited samples on Si substrate the PL signal was collected from the side of the incident laser and a long wave pass filter was used to avoid laser reflections. In both cases, the samples were positioned in an XYZ stage and different samples were excited at the same incident and detection angles, in order to get confident comparative measurements of absolute PL intensity of a set of samples. PL intensity as a function of laser pump energy per area unit at 355 nm was also measured. Samples were excited to reach the saturation level required for the emitted PL intensity. In a two-level model (Wojdak et al 2004), it is expected that the saturation regime of the PL intensity only depends on the quantum efficiency of the samples, and this allows us estimating the absolute increase of the PL intensity. The spot size was around 4 mm and the pump energy per area unit used was below 300 μJcm−2 to avoid heating effects.
For further investigating the processing of photonic signals in the L*NDs, a standard two-wave mixing experiment of the pump and probe beams was conducted with an intensity rate 1:1 with our Nd:YAG laser system. The polarization of the incident beams was linear and the geometric angle between the incident beams was close to 30° just before the interaction with the sample. A total maximum irradiance of 100 MW cm−2 in single-shot mode was used for the experiments. A CS2 sample with a nonlinear refractive index n2 = 4 × 10−14 cm2 W−1 was employed for the calibration of the experimental setup. The rotation of the polarization of the probe beam was measured by using a cross-polarized analyzer behind the sample.
3. Results and discussion
In figure 1, the schematic structure of amine-functionalized NDs is presented. The suggested chemical reactions of amine NDs functionalization with amines have been proposed before (Alzate-Carvajal et al 2016).
Figure 1. Schematic chemical structures of organic amines attached to NDs surfaces: (a) ND-AF, (b) ND-AP, and (c) ND-ODA.
Download figure:
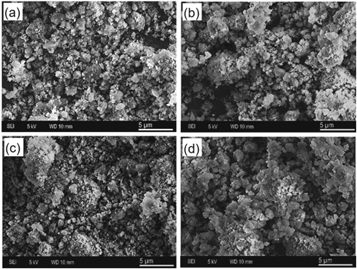
Standard image High-resolution imageComparative SEM images of NDs pristine and functionalized (ND-AP, ND-AF, and ND-ODA) are shown in figure 2. Figure 2(a) revealed large agglomerates of NDs of 20–30 nm size. Dried NDs could be re-aggregated due to capillary forces pulling the individual nanoparticles together; also, attractive van der Waals forces promote the mentioned process as previously reported (Mochalin et al 2012). Organic amine functionalization altered in an evident way the morphology of ND-ODA samples (figure 2(b)), and larger agglomerates of about 1–5 μm were formed due to the stronger inter-particle interactions caused by the covalent functionalization with long hydrophobic radicals of ODA. The aggregation of NDs can be prevented by ultrasound treatment in NaCl solution (Bondar´ and Puzyr´ 2004), or by centrifugation.
Figure 2. SEM images of: (a) commercial pristine NDs powder, (b) ND-AF, (c) ND-AP, and (d) ND-ODA samples Scale bar: 5 μm.
Download figure:
Standard image High-resolution imageRepresentative TEM images of our NDs samples are shown in figure 3. The pristine NDs particle average size was around 4.2 nm (figure 3(a)). Figure 3(b) reveals the crystalline structure of pristine samples (Fayos 1999); the distance between planes (3.6 Å) corresponds to the (110) orientation of diamond (sp3 hybridization) of cubic shape (Barnard 2009). The simulated diffraction pattern obtained and observed using the Fast Fourier Transform (FFT) in DigitalMicrograph software confirms an oriented array of the core and two points of diffraction.
Figure 3. TEM images and histograms of NDs samples before amine modification (a); (b) zoom and corresponding simulated diffraction pattern; (c) ND-AF; (d) ND-AP; (e) ND-ODA.
Download figure:
Standard image High-resolution imageHowever, some dispersion of the brilliant points suggests the presence of an amorphous shell (sp2 hybridization) characteristic of the surface of NDs. The amine-functionalized samples particle sizes were slightly increased in all cases and the biggest particles (of 7.2 nm) observed for ND-ODA samples (figure 3(e)) are strongly dependent on the amine structure attached to NDs surfaces as revealed in SEM images. For ND-ODA the increase in the size of the agglomerates may be due to the interactions between the particles, covered with long aliphatic chains, and the interaction of woolly spheres.
In the ND-AP sample, the intensity was increased. This can be attributed to an excimer or oxidative debris formation after the NDs functionalization with aromatic amines (AF and AP) due to π-π interactions of poly-aromatic fragments (Rourke et al 2011). This effect has been described in graphene oxide (GO) studies (Douda et al 2019b). This type of agglomerate can change average size, thus, resulting in a modification of the optical properties of amine-functionalized NDs.
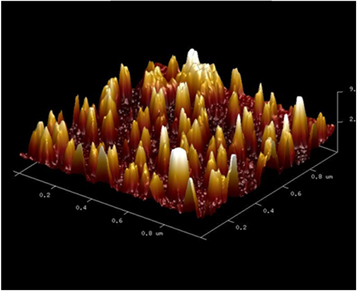
The lecithin liposome average size was evaluated by the Cryo-TEM micrograph and Tapping-mode AFM micrograph and corresponds to 80–200 nm. The respective images are presented in figures 4 and 5, respectively.
Figure 4. Cryo-TEM micrograph of lecithin liposomes.
Download figure:
Standard image High-resolution imageFigure 5. Tapping-mode AFM micrograph of lecithin liposomes.
Download figure:
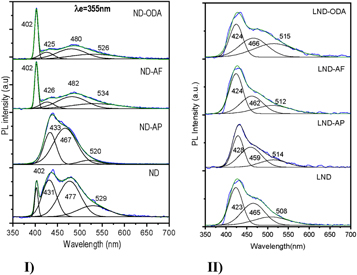
Standard image High-resolution imageThe comparison of studied PL spectra of NDs and amine-functionalized samples, before and after functionalization, is illustrated in figure 6(I), and the same samples after 'green' liposome encapsulation in figure 6(II).
Figure 6. Normalized PL spectra excited at 355 nm in solutions of: (I) NDs; (II) LNDs.
Download figure:
Standard image High-resolution imageThe NDs samples produced a broadband PL spectrum in the range of 380–700 nm excited at the ultraviolet wavelength (355nm). The deconvolution of this band showed four peaks at 402, 431, 477, and 527 nm (PL1, PL2, PL3, and PL4, respectively), see figure 6(I), and table 1. The intensities of pure amines were extremely low in comparison to typical NDs samples.
Table 1. PL peaks positions for NDs before (ND, ND-ODA, ND-AF, ND-AP) and after (LND, LND-ODA, LND-AF, LND-AP) liposome encapsulation.
| Sample | PL2, nm | PL3, nm | PL4, nm |
|---|---|---|---|
| ND | 431 | 477 | 529 |
| LND | 423 | 465 | 508 |
| ND-ODA | 425 | 480 | 526 |
| LND-ODA | 424 | 466 | 515 |
| ND-AF | 426 | 482 | 534 |
| LND-AF | 424 | 462 | 512 |
| ND-AP | 433 | 467 | 520 |
| LND-AP | 428 | 459 | 514 |
Except for ND-AP, in all functionalized samples, the first peak (at 402 nm), which tentatively corresponded to the diamond core emission, did not change its position. However, the adjacent peaks (PL2–PL4) show variations in all samples. The peak at 420 nm is expected to correspond to volume defects in NDs, and the peak at 530 nm to –COOH, or –C–O–C functional groups. However, this peak can also be attributed to: (i) structural disorder on the diamond surface; (ii) presence of carbon in the threefold-coordinated state; (iii) dangling bonds on the surface of the nanoclusters, and (iv) intrinsic defects of a single–crystal diamond ( Kompan et al 1997), and/or, to the presence of an amorphous sp2 shell.
In the PL spectra of NDs functionalized with 1-aminopyrene (ND-AP) the first peak at the blue region is not present. The subsequent three peaks show hyposochromic s hift from 431 nm to 433 nm, from 477 to 467 nm, and the last one from 529 to 520 nm, if compared to pristine NDs spectra. For ND-AF samples the second peak has a significant hyposochromic shift of energy (from 431 to 426 nm), and for the next two peaks the bathochromic shift from 477 to 482 nm, and 529 to 534 nm. The ND-ODA samples show a hyposochromic shift of the second and fourth peaks (from 431 to 423 nm, and 529 to 526 nm, respectively), and the bathochromic shift (to lowest energy) of the third peak from 477 to 480 nm; it corresponds to a slight intensity increase in comparison to pristine NDs spectra.
Also, the PL spectra for non-encapsulated NDs samples measured in ethanol solutions demonstrated the quenching effect of the solvent (not presented here), with the intensities of the emissions several orders of magnitude smaller, and all observed energies presented the red shift for functionalized samples in comparison to pristine NDs spectra.
Liposome encapsulated (by the 'green' method) amino-functionalized NDs samples showed hyposochromic effect for all samples (figures 6(II) and 9, table 1). The first peak at 402 nm disappears, and the second one emerges at 423 nm. Also, the PL intensity for liposome-encapsulated samples was enhanced in comparison to non-encapsulated NDs, with the exception to LND-AP. It is worth noting that the same effect has been observed in NDs samples encapsulated in liposomes prepared by the conventional method. Also, the spectral characteristic of the conventionally prepared liposomes keeps unchanged when they are deposited on silicon substrates, which is important for developing light-emitting and optoelectronics devices.
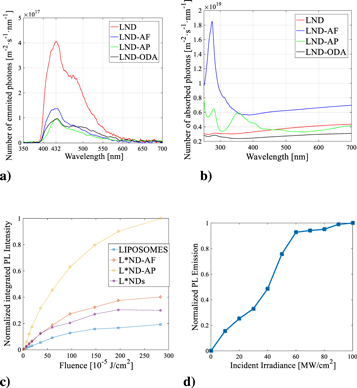
A direct determination of the quantum yield in the samples was obtained by measuring the number of emitted photons (see figure 7(a)) and the number of absorbed photons (figure 7(b)). The quantum yield for the different samples corresponds to the following values: LND = 0.1185, LND-AF = 0.0198, LND-AP = 0.0324 and LND-ODA = 0.0489. These results are in good agreement with previous quantum yields reported in NDs (Bradac et al 2019).
Figure 7. (a) Emitted photons of PL spectra excited at 355 nm of LNDs, LND-ODA, LND-AP and LND-AF; (b) Absorbed photons related to the UV-VIS spectra of LNDs, LND-ODA, LND-AP and LND-AF (c) Normalized integrated PL intensity of pu re Liposomes, L*NDs, L*ND-AP and L*ND-AF as a function of laser energy per unit of area; (d) Non- linear PL emission exhibited by L*NDs.
Download figure:
Standard image High-resolution imageAlso, the PL of NDs functionalized with amines and encapsulated in liposomes by the conventional method was analyzed as a function of the incident laser pulse energy. The PL intensity from L*ND-AP was higher in respect to pure liposomes, pristine L*NDs, and other amine-functionalized samples.
At lower laser energy the PL increases linearly for all samples. However, the slope of the plot is higher for the functionalized samples in comparison to L*NDs (figure 7(c)). This indicates that the excitation cross-section of the functionalized samples has increased when comparing them to the pure liposome and the NDs. In particular, the L*ND-AP sample has a more pronounced slope, almost 6-fold enhanced in contrast with the L*NDs samples. The excitation cross-section for L*ND-AF and L*NDs samples have almost the same value but enhanced by a factor of 2 with reference to the pure liposome samples. The PL enhancement at the saturation regimen pointed out that for the L*ND-ODA sample the PL quantum efficiency increases by a factor of about 4 in comparison to L*NDs. In contrast, t he enhancement factor of the PL quantum efficiency for L*ND-AF and L*ND-AP samples are about twice and three times higher than L*NDs.
Saturation in the PL emission as a function of the incide nt irradiance was measured between 60 MW cm−2 and 100 MW cm−2 in the L*NDs, as shown in figure 7(d). In the nonlinear regime, the results show no modification in the location of the peaks of emission, as well as a lack of photothermal or ablation actions. Therefore, it can be stated that an induced transparenc y is present under excitation of the sample by picosecond pulses.
Multi-photonic effects in L*NDs indicate the possibility to fully occupy quantum levels by nonlinear optical signals in the sample. Thus, the potential control of information and energy transfer in NDs can be considered for encoding information in light by all-optical modulation mechanisms assisted by encapsulation processes.
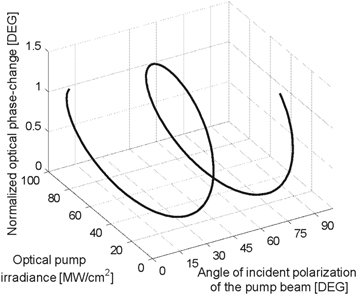
Two-wave mixing experiments were undertaken to explore any potential induced birefringence on the propagation of the beams interacting in the L*NDs samples studied. The evolution of the phase-change in the sample as a function of the estimated nonlinear refractive index n2 = −2 × 10−13 cm2/W for the L*NDs is shown in figure 8.
Figure 8. Optical phase-change induced by a two-wave mixing in an optical probe signal for L*NDs.
Download figure:
Standard image High-resolution imageFigure 8 clearly describes that the incident irradiance of the pump beam can modify the optical phase in the probe beam in our sample. A polarization-resolved effect can be also observed regarding that the superposition of the incident beams is able to generate an irradiance interference pattern that automatically modulates the polarization and the phase of the probe beam in the interaction. Light controlled by light in a two-wave mixing configuration can be useful for developing quantum functions in an ultrafast temporal regime.
In this work, a clear indication of the modification in PL and nonlinear optical phenomena derived by the encapsulation of NDs was analyzed. The results highlight the evolution of the nonlinear optical signals induced in encapsulated and functionalized NDs samples. The NDs seem to be dominantly responsible for multi-photonic effects in all cases in our study. But functionalization and encapsulation can play an important role in the potential functions of this nonlinear response. We report that optical phase-changes generated by a two-wave mixing can be employed for immediate all-optical applications. The understanding of simple third-order optical nonlinearities proposed in this manuscript can be a base for developing future research of nanostructures that can be related to instrumentation and sensing functions.
The effect of wavelength-shift of emission energy and intensity after NDs encapsulation in liposomes prepared by both methods (green end conventional) and measured in solution or silica surface cannot be attributed to the presence of a solvent, silica, or the method of liposome preparation. Thus, amine functionalization offers surface modification due to amidation reactions (Astuti et al 2014, Alzate-Carvajal et al 2016, Alzate-Carvajal et al 2018, and references therein). Also, the studied aromatic and aliphatic amine-functionalized samples together with highly oxidized fragments (oxidative debris), provoke planar conjugated domains (Rourke et al 2011, Kumar et al 2012), or formation of the interaction of woolly spheres, and produce new effects and tension forces, and effectively reduce the sp2 surface defects. These effects are likely to be involved in the change of the PL properties observed in figure 6(I).
On the other hand, as it was reported by Mona et al 2012, the PL enhancement may be caused by the combination of the intrinsic PL of NDs core with trapped excitation originated from surface defects (disordered carbon, graphite, and other sp2 hybridized peculiarities) that can be transferred to the set of energy levels. From figure 9, a comparative PL modification in NDs can be visualized, considering that amine-functionalization and liposome encapsulation may reduce some surface defects and induce additional levels in the band gap in comparison to pristine diamonds, and doped NDs. Also, Fokin and Schreiner (2009) concluded that the band gap in NDs is determined only by the nature of dopant rather than the size in the cage. This is due to the dominant contributions of the atomic orbitals of the dopant into the HOMOs and the LUMOs.
Figure 9. Graphical representation of PL modification of diamonds upon their structural modifications among bulk diamonds, NDs, amine-functionalized and liposome-encapsulated NDs, reported in this work.
Download figure:
Standard image High-resolution imageIt is worth mentioning that other important phenomena are suitable to be assigned to the encapsulation of the NDs in liposomes (figure 6(II)). For instance, when NDs are encapsulated in a lipid bilayer, additional compressive stress can be incorporated on the NDs, and this results in a modification of their optical pr operties. The liposome bilayer width in our case is estimated to be around 5 nm but it may vary with the type of phospholipids used. The hydrophobic particles of small size, like N Ds (4–6 nm), may be enclosed on a lipid bilayer because of their hydrophobicity. Consequently, a larger additional tension, and respectively, an increased emission energy can be expected (figure 9). Also, to be considered, soybean lecithin phospholipids in liposomes are charged molecules and induce the electric charge in the surface area of NDs, which in turn produce higher energy gap changes for applied NDs.
Amine-functionalized and liposome-encapsulated NDs show promising properties for improving quantum yields and tuning PL of NDs for their application as multifunctional biomedical imaging fluorescent markers. The mechanism of the PL enhancement of NDs functionalized with amine molecules is not clear yet and engineering their respo nse is still a challenge. However, there is experimental evidence on altering the transition-energy levels in nanostructures by using organic amines, liposomes, and solvents for NDs PL modification. Different organic amines have been eval uated as PL tuning agents and the responses are strongly dependent on the hydrocarbon structure and functional groups on NDs surfaces.
The surface-to-volume ratio in nanomaterials is a fundamental advantage for designing nanoscale effects because they are strongly sensitive to their size, the presence of impurity atoms and the shape of NDs nanostructures. Any slight modifications of their surface may produce considerable modifications in their emission properties. Changes in the atomic dipole moment can be explained by the electromagnetic field allowed near and out of the surface of nanoparticles. And an important influence can be expected from the surrounding of NDs in the electronic dynamics governing the physical mechanisms responsible for their photonic phenomena. Particularly, the participation of functionalization processes in NDs may derive in changes in population loss through radiative and non-radiative processes of the upper quantum level of excitation, and also a modification in the polarization loss that describes the evolution of a nanostructured system. Analogically with the semiconductors, nanometric diamonds may show a band gap modification effect (Wu et al 2010, Perevedentseva et al 2011, Mochalin et al 2012, Osipov et al 2020). It is well known, that due to the effective particle size reduction, the quantum confinement effect can increase (Kryshtab et al 2012, Polupan et al 2017), and eventually, a reduction in semiconductor nanoparticles effective sizes may be derived from liposome encapsulation (Douda et al 2018, and 2019a). These considerations allowed us to contemplate encapsulated NDs with PL tuning advantages. The original approach combining nanotechnology and solid-state chemistry methods opens the way to the building of innovative materials for integration and multifunctionality. These are two fundamental points in multidisciplinary fields of applications such as electronics, telecommunications, optics, environment, and biomedical functions (Liu et al 2013, Torres Sangiao et al 2019).
The main value of our work is based on the evaluation, for the first time, of the PL phenomena exhibited by functionalized NDs and their modification by the liposome encapsulation. The PL spectra of the NDs were measured before and after their encapsulation in liposomes, prepared by two comparative methods starting from soybean lecithin. These emission effects can be analyzed by a straightforward PL technique without any special preparation, in contrast with the common conditions currently mandatory in clinical laboratories (Patra et al 2018, Bamburowicz-Klimkowska et al 2019, Torres Sangiao et al 2019). It can be considered that theragnostic liposomes containing NDs with enhanced optical properties are quite viable, due to their biocompatibility and potential assistance in fluorescence microscopy. Besides, encapsulated NDs-based samples can be a useful tool for sensing and instrumentation of signals in biomedical sciences.
4. Conclusions
Pristine and amine-functionalized NDs have been encapsulated in soybean liposomes prepared systematically by two different methods in this work by means of PL studies. The structure of applied amine promotes different modifications in effective size and luminescence response of NDs. The liposome encapsulation increases the optical response of both: the intensity and maximum emission energy. The obtained optical function modifications in the synthetized NDs-liposome hybrids may play a crucial role in the development of theragnostic liposomes and all-optical sensors, opening the possibility for their usefulness in other biomedical applications.
Acknowledgments
The financial support from the Instituto Politécnico Nacional (grants SIP-IPN, Mexico), Universidad Nacional Autónoma de México (grant DGAPAIN101118), and Consejo Nacional de Ciencia y Tecnología (postdoctoral grants 711124 and 2019-000019-01NACV-00297) was fundamental for the development of this work. The authors kindly appreciate the scientific support from Dr V A Basiuk (Instituto de Ciencias Nucleares, UNAM) for sharing the functionalized ND samples, and Victor Meza Laguna for SEM images. Authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación-SAI, Universidad de Zaragoza, and LMA microscopy laboratory at INA for the facilities in TEM.
The data that support the findings of this study are openly available under request to the corresponding author.