Abstract
Abstract
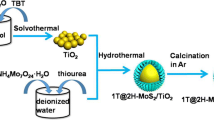
MoS2-deposited TiO2 hollow spheres were synthesized successfully under mild temperature and autogenous pressure. The hydrothermal technique was adopted for the synthesis of the TiO2 hollow microsphere, followed by a photodeposition technique for the deposition of MoS2. The physical and chemical nature of the samples was characterized using X-ray diffraction, energy-dispersive X-ray spectroscopy, scanning electron microscopy, photoluminescence spectroscopy, XPS and UV–vis spectroscopy. In an aqueous medium under the influence of light, the characterized samples were used in the production of hydrogen via photocatalysis. The increase in the formation of hydrogen content during photocatalysis confirms the successful generation and the benefits of the photogenerated carriers. With an increase in the MoS2 content, there is an incredible change in the photocatalytic performance. The resultant is due to the free moment of the holes and electrons and lessening in charge recombination centres formed as a result of the nano-heterojunction linking between MoS2 and TiO2. A more significant photocatalytic production of hydrogen was achieved using 50 MST sample i.e. 106 μmol−1 g−1 beyond which it tends to decrease with an increase in MoS2 content.
Graphic abstract

Similar content being viewed by others
Introduction
Generally, resources can be considered as those materials/products that satisfy or meet human requirements. The boom in technology and industrialization has led to the overutilization of economically important energy resources such as fossil fuel and petroleum which are nonrenewable. The overexploitation of these energy resources ultimately results in an energy crisis affecting the economic development of a nation. Depletion of the natural resources leads to the deterioration of the environment, causing global warming, greenhouse effects, acid rain and health hazards [1, 2]. For the sustenance of life and to maintain a healthy environment, natural resources have to be conserved. Conservation of these resources by finding a suitable alternative is in practice. Efforts are being made on the utilization of renewable resources such as solar, wind and water current. Meanwhile, material scientists and environmentalists are paying attention to recycling and to reusing unwanted/waste materials as an alternative source of energy. The development of the materials that can convert waste material or recyclable material into a useful form of energy is a challenging field. Attempts have been made for the treatment and recycling of wastewater, which is a practical application. However, very little work has been performed in utilizing wastewater for the production of energy. Fabricating of the designer hybrid photocatalytic material with enhanced photocatalytic property and their application in the production of hydrogen by dissociation of the water molecule is a matter of interest in recent years [3,4,5,6,7]. Application of these photocatalysts for the conversion of wastewater which is not fit for domestic as well as industrial purpose into a useful form of energy will reduce the burden on nonrenewable energy forms to a certain extent.
In semiconductor photocatalysis, titanium dioxide (TiO2) is a topic of debate. TiO2 as a photocatalyst is applicable in various fields such as hydrogen production, wastewater treatment, water splitting, dye degradation, and carbon reduction [8,9,10,11,12,13,14,15,16]. The propertiesof TiO2 such as strong oxidizing agent, high stability, abundantly availability, low-cost and its non-toxic nature makes it a stable candidate in semiconductor photocatalysis [14, 17,18,19]. TiO2 exists in three forms, namely, anatase, rutile and brookite. Among these, anatase is an indirect bandgap semiconductor and has higher photocatalytic activity compared to rutile and brookite which are direct bandgap semiconductors [20]. The life span of photoexcited electrons and holes in anatase is comparatively high compared to that of rutile and brookite [5]. However, as a catalyst, anatase TiO2 has its limitations in the application. The bandgap possessed by the anatase TiO2 is 3.2 eV which is not well matched with visible light excitation [21]. As a result, the photoexcited electrons and holes recombine rapidly leading to less photocatalytic efficiency.
It is reported that TiO2 semiconductor along with other metal oxides and sulphides has been proven to be a capable material in photocatalysis under both ultraviolet and visible light. Metal oxides and sulphides such as ZnO, MoO2, CeO2, ZrO2, SnO2, CdS, ZnS, Ag, compounds of carbon, along with TiO2, form heterojunction which reduces the recombination of photoexcited electron and hole pair [22]. Among sulphides, efforts have been made for the development of composite material using MoS2 along with TiO2 using different techniques. Techniques adopted for the fabrication of these composites are hydrothermal, solvothermal, in situ growth method, chemical vapour deposition and calcination [23,24,25,26,27]. Earlier, molybdenum disulphide (MoS2) was used in composite materials, for the reason that it possesses a characteristic layered structure along with tough interlayer covalent bonds alienated by a weak van der Waals gap [27]. This van der Waals gap is considered as a significant reason for its application as catalyst. The layers of the MoS2are similar to that of the layers which are exhibited in graphene, and hence in recent years MoS2 has been used extensively used [28]. In the MoS2sheet, each sheet is fashioned by the combination of three layers. The atoms of molybdenum (Mo) are placed in the centre layer, whereas the layer consisting of sulphur (S) atom envelops the Mo atom from both the sides [28, 29]. Even, MoS2 possesses similar properties to those of TiO2, such as high oxidizing action, high hardness, non-toxicity, low cost, and high stability and reliability. These features and the direct bandgap possessed by Mo, which is approximately 1.8 eV, has attracted the mind of the researchers to make use of this visible-light active photocatalyst in enhancing the efficiency of another semiconducting photocatalyst. Attempts have been made for the development of TiO2/MoS2 composite material where 2D MoS2 materials are embedded onto 1D TiO2nanoparticles and their application in the evolution of hydrogen, which has proven to have shown a satisfactory result [30,31,32]. In the present article, we report the simple technique for the fabrication of TiO2/MoS2 composite material. Processing of this material involves a mild hydrothermal condition for the formation of TiO2 hollow microspheres, followed by a photodeposition method for the deposition of MoS2 onto the TiO2 hollow microspheres and the application of this composite material in the generation of hydrogen.
Materials and methods
All the reagents used in the present work were of laboratory grade and used without further purification. Ranbaxy Chemicals Co. Ltd., Sigma-Aldrich and Loba Chemicals Co. Ltd. supplied hydrochloric acid (HCl), titanium butoxide (C16H36O4Ti), ammonium tetrathiomolybdate ((NH4)2MoS4) and ethanol. Throughout the experiment, distilled water was used.
Hydrothermal synthesis of TiO2 hollow microspheres
Commercially available C16H36O4Tiis was used as a starting material. Into a 100 ml Teflon liner, 25 ml of 4 M HCl was taken. 3.4 ml of C16H36O4Ti was added to the Teflon liner dropwise with continuous stirring using a magnetic stirrer. The stirring process was carried out for 2 h. After stirring, the Teflon liner was closed tightly and placed in an autoclave, and heated at the 180 °C for 24 h. The hydrothermally treated autoclave was cooled suddenly to arrest the morphology developed by the crystals in the set experimental condition using a compressed air jet. The liner was removed, and the solution inside the liner was discarded to separate the powder sample. The powder was washed thoroughly using double-distilled water thrice to remove the unwanted compounds. The powder sample was then ultrasonicated to avoid agglomeration. The sample was centrifuged, extracted and dried at a temperature of 35–40 °C in an oven.
Photodeposition of MoS2 onto TiO2 hollow microspheres
The photodeposition of MoS2 onto the TiO2 hollow microsphere was carried out in a double-necked container. In the photodeposition of MoS2 onto TiO2 hollow sphere, 50 mg of the as-prepared TiO2 hollow microsphere was dispersed in a 20 ml mixture of ethanol and distilled water (5 ml + 15 ml). To this solution, a known amount of (NH4)2MoS4 was added. N2 was continuously bubbled through the solution for 20 min to eradicate oxygen. The solution was then exposed to a 300 W Xe lamp for the 1-h duration. The solution was continuously stirred using a magnetic stirrer. The supply of N2 was continued. After the experimental run, the products were separated by centrifuging, washed thoroughly with distilled water and dried in an oven at 60 °C. Based on the weight ratio of MoS2 added, the sample obtained was named as 0 MST, 5 MST, 10 MST, 30 MST, 50 MST, and 70 MST where the numbers represent the wt% of MoS2.
Hydrogen production by photocatalysis
The photocatalytic hydrogen production experiments were performed in a 100 ml three-necked Pyrex flask. The analysis was performed at ambient temperature and atmospheric pressure conditions. All three necks of the flask were sealed with a silicon rubber septum. As a source of illumination, 350 W xenon arc lamp with a UV cutoff filter (λ ≥ 400 nm) was positioned 20 cm away from the reactor which triggers the photocatalytic reaction. The strength of light exposure on the flask was measured as 180 mW cm−2. In a distinctive photocatalytic experiment, 50 mg of catalyst was diffused in 80 ml of a mixed aqueous solution having 0.35 M Na2S and 0.25 M Na2SO3. Through one of the rubber septums, the system was bubbled with nitrogen for 40 min to remove dissolved oxygen and to create an anaerobic condition. During irradiation, the solution was continuously stirred to keep the photocatalyst in suspension. 0.4 ml of gas was intermittently sampled through the septum, and hydrogen was analyzed by a gas chromatograph (Shimadzu GC-14C, with nitrogen as a carrier gas) equipped with a 5 Å molecular-sieve column and a thermal conductivity detector.
Instruments and characterization
X-ray diffraction (XRD) pattern of the powder samples was recorded by the RigakuMiniflex X-ray diffractometer, model IGC2, Rigaku Co. Ltd., Japan. The 2θ range was positioned between 20° and 80°. The detection of the crystalline phase was done by matching up with JCPDS using PCPDFWin version 2.01. The Fourier transform infrared spectrometry (FT-IR) spectra were recorded using JASCO-460 Plus, Japan. The morphological features of the as-prepared samples were examined by a high-resolution scanning electron microscope model HITACHI S-4200 and model JEOL (FESEM). Energy-dispersive X-ray spectroscopy (EDX) was used to envisage the deposition of MoS2 onto the hollow sphere. UV–visible spectrophotometer, model UV-2550, Shimadzu, Japan, was employed to witness the UV–visible absorbance spectra for the dry pressed samples in the presence of BaSO4 as a standard. Fluorescence spectrophotometer was adopted to determine the photoluminescence (PL) spectra of the samples at room temperature. X-ray photoelectron spectroscopy (XPS) measurements were performed using an ultrahigh vacuum VG ESCALAB 210 electron spectrometer having a multichannel detector.
Results and discussion
X-ray diffraction studies
The powder XRD patterns MoS2, 0 MST and 10 MST are given in Fig. 1a. For comparison, standard XRD patterns of MoS2 and TiO2 are given in Fig. 1b. The identification of the crystalline phase of these samples is performed by comparing the obtained data with the JCPDS file (PCPDFWIN-2.01). The XRD patterns for both 0 MST and 10 MST match with PDF- 21-1272, representing a tetragonal system, which fits into the space group I41/amd [33].
The XRD patterns further validate that the samples consist of pure anatase phase. In the 10 MST sample, the development of the new peaks corresponding to MoS2was noticed. This result suggests that the MoS2 was well deposited onto the surface of TiO2 hollow spheres. There was a very slight shift in the peak position confirming that the MoS2 was filled only on the surface of the TiO2 and did not enter the crystal lattice. The result further states that the deposition of MoS2 onto TiO2 hollow microspheres did not affect the structural morphology of the crystal. For comparison, the XRD pattern of the MoS2 was recorded, which matches with PDF 37-1492 [34]. Even the peaks developed in the XRD pattern of the 10 MST sample matched well with the XRD pattern of MoS2 (002, 100 and 110 planes). The deposition of MoS2 onto the TiO2 hollow spheres leads to the formation of a heterojunction between MoS2 and TiO2 particles, which further separates the recombination of electron–hole pairs, leading to the enhancement in the photocatalytic activity.
SEM studies
The shape and the structural features of the fabricated MoS2-deposited TiO2 hollow microspheres were analyzed through SEM micrographic studies (Fig. 2). In Fig. 2a–c, one can witness that the synthesized crystals are arranged in a sphere shape, ranging approximately 5–7 μm and having a cavity within it. In Fig. 2a, the arrangement of TiO2 nanocrystals in radiating patterns to form a sphere can be visualized. On a closer look at the micrograph, one can observe the deposition of foreign materials onto the TiO2 hollow microsphere, which might be the deposition of MoS2 (Fig. 2b and c). Figure 2d, clearly demonstrates that a single hollow microsphere comprises of numerous nanocrystals which appears almost tetragonal in a shape whose size varies and is less than 0.1 mm thickness. The SEM studies further confirm that the hollow microspheres can be synthesized in the absence of templates or surfactants employing the hydrothermal technique in the present set condition. It is well known that in a hydrothermal run, the crystals are formed by dissolution and recrystallization process. As temperature increases, titanium gets dissolved. As it reaches the top condition, it gets re-crystallized to form nanocrystallites. These nanocrystallites further undergo an Ostwald ripening, forming hollow microspheres [35]. In Ostwald ripening, the crystallites present in the inner part are circularly arranged, are smaller in size or lighter and thinner compared to those present in the outer region. These tiny crystallites get dissolved, and re-deposited on the outer parts, resulting in the formation of the hollow cavity at the centre forming hollow spheres. The cross section of these hollow spheres demonstrates that the inner layer is occupied by smaller crystals and the outer with the larger crystals, and the arrangement of these crystals in a radiating pattern is given in Fig. 2a.
Energy-dispersive X-ray spectroscopy studies
The element composition and the elementary mapping of the 5 MST sample are given in Figs. 3 and 4. Figure 3b and c gives the elementary mapping verifying the presence of Ti in addition to O. The elementary mapping for the deposition of Mo is shown in Fig. 3d. From Fig. 3, it is clear that the synthesized microspheres sample is dominated by TiO2, with a trace of Mo decorated on it. The EDX spectrum for 5 MST sample was taken from the selected point, keeping SiO2, Al2O3, Ti, and Mo as reference samples. The point analysis showed the strong signals of Mo, Ti, O and Al (Fig. 3). The Al peaks were observed in the EDX analysis, since the aluminium foil was used as the holder. These results indicate that Mo, Ti and O are the primary constitutive elements of the TiO2 hollow sphere deposited by MoS2. The presence of carbon at 0 eV is due to the use of the carbon-coated grid during analysis. The details of the elements present in a particular point of the hollow microsphere during the investigation are given in Table 1.
FT-IR spectroscopy studies
FT-IR analysis was performed for MoS2, 0 MST and 10 MST samples to determine the functional group of the synthesized compound. The spectra obtained are given in Fig. 5. The band in the region 472 cm−1 corresponds to the Ti–O vibrations [36]. The Mo–S band vibration was observed in the region around 920 cm−1 [37]. The band in the region 1700 cm−1, corresponding to C–O formed due to the absorption of CO2 from the surrounding environment [38], and the bands lying in between 3000 and 3500 cm−1 correspond to the O–H vibration caused due to the absorption of moisture [39]. Both the Ti–O and Mo–S vibration were observed in the 10 MST samples, further confirming the deposition of the MoS2 onto the TiO2 hollow spheres.
PL spectral studies
The PL spectra for the selected samples are given in Fig. 6. The PL analysis will be of assistance in knowing the efficiency of charge carrier entrapping, immigration and shift of electrons. The deposition of MoS2 onto TiO2 hollow microspheres has a significant outcome on the PL intensity (Fig. 5). There is a reduction in the PL intensity of the samples with an added amount of MoS2 content. The PL emission spectra of 0 MST sample showed the existence of several peaks appearing at wavelength approximately 419, 438, 450, 468, 481, 494, 528 and 540 nm. The wavelengths of these peaks are equivalent to 2.9, 2.8, 2.7, 2.6, 2.57, 2.51, 2.3 and 2.2 eV. Similar peaks were developed with the 5 MST and 10 MST samples. In 0 MST, the emission band at 419 nm is considered as a stronger band. However, the deposition of MoS2 suppressed the emission at 419 nm. Further, it was observed that the emission band at 438 nm had stronger emission in the rest of the samples after the deposition of MoS2, which resulted due to the disturbances caused by the filling of MoS2 onto TiO2. In the samples, when the MoS2 content was increased from 5 to 10 wt%, there was a drop in the PL intensity. It is worthy to note that the intensity of PL depends on the colour of the sample subjected to PL spectral studies. The darker the sample, the lesser is the intensity, which is caused due to the black body absorption of the spectrum. In this study, no comparison was carried out for the MoS2 sample; the colour of MoS2 is blackish and its excitation occurs beyond 650 nm. It is noteworthy that if the rate of recombination of electron and holes is less, then there is a reduction in the PL intensity. Hence, it can be assumed that the reduction in the PL intensity might be due to the black body absorption of light or to the formation of a heterojunction between TiO2 and MoS2 particles. In a hybrid semiconducting material, the formation of heterojunction decreases the recombination of the electron–hole pair, thereby enhancing the photocatalytic ability of the photocatalyst. The explanation behind this is that the PL emission is formed as a consequence of the recombination of light-induced electrons and holes pairs. The higher the intensity of the PL spectra, the lesser is its photocatalytic activity. In the present study, the 10 MST sample shows lower intensity compared to the 5 MST and 0 MST samples, suggesting that the deposition of MoS2 onto the TiO2 hollow microsphere has a major effect in reducing the recombination of electrons and holes and has all possibilities of performing high photocatalytic activity. The quick electron transfer between MoS2 layers and TiO2 crystals increases the quantum efficiency, thereby supplying more photogenerated electrons which further participate in photocatalysis.
UV–vis diffuse reflectance spectra
The UV–vis absorption spectral studies for the as-prepared samples were performed (Fig. 7). As per our examination report, in the bare TiO2 hollow microspheres (0MST), the absorption edge lies at 411.4 nm which is almost agreeable with the bandgap absorption of TiO2 (380 nm). In the present study, the bandgap obtained for 0 MST is 3.01 eV. MoS2was photodeposited onto this sample. The photodeposition of MoS2 onto 0 MST leads to the prime increase in the absorption of the sample beyond 411.4 nm. Nevertheless, with a varying MoS2 concentration, there is a slight increase in the redshift absorption of the samples by 0.01–0.04 eV. The small shift in the absorption of the sample implies that the MoS2 particles did not intrude into the crystal lattice of the TiO2 hollow microsphere and were deposited on it, which is in agreement with the EDX and XRD report (Fig. 7a). When MoS2 is deposited onto TiO2, it may create a lattice disparity which forms a defect in the crystals. Further, to make out the importance of MoS2 in altering the bandgap energy of TiO2, the bandgap energy of the chosen samples cited above was estimated using the Tauc plot and by extrapolation of the linear slope of photon energy (Fig. 7b). The modification in the bandgap energy of the sample relating to the amount of MoS2 added corroborates the effective deposition of MoS2 onto the TiO2 hollow microspheres. It is worth mentioning that the bandgap energy for the representative samples is as follows: Bg of 0 MST is 3.013 eV < 5 MST, which is 3.047 eV < 10 MST, which is 3.056 eV. Our studies direct to the conclusion that the altering in the bandgap energy, even to a small amount, is most likely to be the outcome of the formation of new electron levels of Mo ions in the TiO2 band structure, which further confirms the active deposition of MoS2 onto TiO2 hollow microspheres. The deposition of MoS2 onto the TiO2 hollow microspheres may favour free movement of electrons as well as holding back the recombination of photogenerated electron and hole pairs.
X-ray photoelectron spectroscopy (XPS) studies
The XPS analyses for the 0 MST, 10 MST and MoS2 samples were performed to know the chemical state on the surface of the materials synthesized. The Ti2p spectra of the synthesized compound show two bands located at binding energies of 458.9 and 464.8 eV in 0 MST and 459.1 and 464.8 in 10 MST samples, which are assigned as Ti2p1/2 and Ti2p3/2 spin-orbital splitting photoelectron in Ti4+ state (Fig. 8a) [40]. In Fig. 8b, the XPS spectrum represents the deconvoluted peaks of O1S. The peak positioned at 531.5 eV for 0 MST and 531.3 eV for 10 MST sample is attributed to the oxygen anions of the TiO2 lattice, whereas, in the peak positioned at 533.3 eV of 10 MST sample, we can notice a slight increase in the peak intensity compared to the peak of the 0 MST sample positioned at 533.5 eV, which can be attributed to the chemically absorbed oxygen onto the surface. In Fig. 8c, the binding energies for Mo 3d3/2, Mo 3d5/2 are 241.3 and 241.8 eV corresponding to MoS2sample and 241.4 and 241.9eV corresponding to the 10 MST sample, which confirms the existence of Mo3+ and Mo4+. The presence of Mo3+ ions depict the development of S defects in MoS2. The high resolution S 2p spectrum obtained for the 10 MST sample shows that the chemical states of S are overlapping (Fig. 8d). In the 10 MST sample, the binding energy observed at 161.7 eV is assigned to S 2p. The development of this peak results from the deposition of Mo–S onto TiO2. Even the peaks at the region 162.5 and 169.2 eV were observed. These peaks originated due to the presence of S.
Photocatalytic H2 production activity and its mechanism
The amount of the hydrogen evolved during photocatalysis using TiO2 hollow microspheres and MoS2-deposited TiO2 hollow microspheres are shown in Fig. 9. Based on the results obtained, one can infer that under visible light irradiation using 0 MST, no H2 production was detected. Similarly, the test for hydrogen using MoS2 as a photocatalyst was conducted. Even in this case, the output/evolution of hydrogen was very less. However, when MoS2 was deposited onto the 0 MST sample, the production of H2 was observed. The production of H2 was considerably increased with the added amount of MoS2. Initially, when 2% MoS2 was deposited onto the TiO2 hollow microsphere (2 MST), the production of hydrogen was observed. In the 2 MST sample, the production of hydrogen was 27 μmol h−1 g−1. Further increase in the percentage of MoS2 content led to an increase in hydrogen production up to a certain extent, i.e. for 5 MST, 10 MST, 30 MST, 50 MST, 70 MST and MoS2, the production of hydrogen was 46, 68, 96, 142, 76 and 20 μmol h−1 g−1. In the present study, optimal photocatalytic activity was achieved using the 50 MST sample, and the rate of hydrogen production using this sample reached 142 μmol h−1 g−1, which is more than five times that of 2 MST sample. Further increase in the MoS2 content to 70 wt% led to the reduction in the photocatalytic activity lesser than that of the 30 MST sample. The blank/dark experiment was also conducted using the above-mentioned samples, and in the absence of light, showed no significant production of hydrogen. The investigation carried in the dark confirms that the formation of hydrogen was due to the photocatalytic property exhibited by the as-prepared samples.
There are few factors which enhance the photocatalytic activity of TiO2 hollow microspheres when MoS2 is deposited onto it. The MoS2 particles deposited onto the 0 MST samples are electro-catalytically very active and hence participate in the reduction of a water molecule [41]. During photocatalysis, these MoS2 particles participate in the overall reaction without undergoing any decomposition. The photodeposited MoS2 forms heterojunction with TiO2. When TiO2 is exposed to light higher than its bandgap energy, under light illumination the electrons are generated in the conduction band (CB), and holes in the valence band (VB). These electron and holes recombine quickly, thereby reducing the efficiency of photocatalysis. When MoS2 is deposited onto TiO2, the heterojunction formed in between them decreases the rate of charge recombination and enhances the ability of the photocatalyst. Normally, the materials of the chalcogenide series undergo decomposition during photoexcitation [42]. However, in MoS2, it is encompassed of Mo 4d orbitals in both the CB and VB due to which the bonding between the Mo and S is firm, and hence during photocatalysis, the MoS2 will not undergo decomposition even after photoexcitation [43]. The deposition of such a high photostable compound onto the TiO2 MSs enhances both their stability and photocatalytic property. This photostability property possessed by the MoS2 favours enhancement of the production of H2. It can also be said that the defect created on the TiO2 crystals due to the deposition of MoS2 favours improvement of the effectiveness of the synthesized samples. The deposited MoS2 is bluish-black. It is well known that black bodies have the affinity to absorb more light. The deposition of MoS2 serves in light harvesting and hence enhances the photocatalytic behaviour of the compound. The morphology of the crystal synthesized also plays a crucial role in enhancing the efficiency of the photocatalyst. The hollow sphere formed by Ostwald ripening consists of a cavity within it. Even the meso/macropores are incorporated between the TiO2 crystals, which are arranged in a typical pattern to form the hollow sphere. This structural arrangement helps in light harvesting by allowing the light to pass through their pores, channels, and the hollow cavity leading to the scattering of light [44]. Scattering of light within the hollow spheres favours the generation of more electron and holes which further participate in the photocatalytic reaction, thereby enhancing the efficiency of the photocatalyst. However, it was observed that with an increase in MoS2 content beyond 50% wt, there is a decrease in the efficiency of the photocatalyst. The decrease in the photocatalytic activity is possibly due to the rise in charge recombination centres formed due to the surplus deposition of MoS2. When the excess MoS2is made to deposit onto the hollow spheres, some of the particles do not make intimate contact with TiO2. These particles get dispersed in the liquid medium. These dispersed particles act as barriers and retard the penetration of light to a certain extent which in turn reduces the photocatalytic process.
The stability and recycling performance of photocatalysts is an essential factor for practical applications. The H2 production performance in a cyclic photocatalytic run was performed to know the stability of the as-prepared sample (Fig. 10). A 50 MST sample was used in the present case. Not much difference was observed in the production of hydrogen after three cycles. The maximum hydrogen production was 872, 882 and 876 μmol h−1 g−1 in all three cycles in 5-h duration. The amount of hydrogen produced in the three cycles confirms the excellent stability of the sample during the photocatalytic process.
Based on the above studies, we proposed a mechanism for the charge transfer between TiO2 and MoS2 during the photocatalytic production of hydrogen (Fig. 11). When UV/sunlight is illuminated on the surface of TiO2, it absorbs light, producing photogenerated electron–hole pairs. On the surface of the photocatalyst, this photogenerated electron and holes can participate either in oxidation or reduction; if not, they can even recombine. When TiO2is exposed to light in the absence of other supportive semiconductor/co-catalyst, these photogenerated electrons and holes immediately recombine reducing the photocatalytic efficiency. When a semiconducting material like MoS2 is deposited onto TiO2, the electrons generated in the conduction band of TiO2 move to the surface of MoS2. The heterojunction formed between the two semiconducting materials creates an intimate contact between them, favouring the free moment of the electron. The as-formed heterojunction reduces the recombination of the electron–hole pair thereby enhancing the efficiency of the photocatalyst. The MoS2 deposited onto the surface of hollow spheres serves as the active site for the production of hydrogen. Onto the surface of MoS2, the migrated/transferred electrons from TiO2 react with the H+ ions adsorbed by the active sites forming hydrogen.
Conclusions
Synthesis of MoS2-deposited TiO2 hollow microspheres was carried out using mild hydrothermal conditions supported by photodeposition. The characterization studies performed states that the MoS2 was efficiently deposited onto the hydrothermally synthesized TiO2 hollow spheres. The deposition of MoS2 enhanced the efficiency of photocatalyst by forming the heterojunction between TiO2 and MoS2, which further reduces the charge recombination. The photocatalytic production of hydrogen using the as-prepared samples confirms the importance of these materials in environmental issues for the production of clean and low-priced energy. We further stressed that the efficiency of the photocatalyst in the production of hydrogen depends on the added amount of MoS2. The stability test performed for this sample exposed that the active catalyst can be reused for more than three cycles, thereby reducing the cost of operation. Overall, we can conclude that the application of MoS2-deposited TiO2 hollow microspheres in the production of hydrogen will be a safe, clean, economical, and environmentally benign technique to overcome the energy crisis and to protect our existing nonrenewable resources.
References
Bilgen, S.: Structure and environmental impact of global energy consumption. Renew. Sustain. Energy Rev. 38, 890–902 (2014). https://doi.org/10.1016/j.rser.2014.07.004
Ayhan, D., Sahin-Demirbas, A., Demirbas, A.H.: Global energy sources, energy usage, and future developments. Energy Sour. 26(3), 191–204 (2004). https://doi.org/10.1080/00908310490256518
Wang, X., Maeda, K., Thomas, A., Takanabe, K., Xin, G., Carlsson, J.M., Domen, K., Antonietti, M.: A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8, 76–80 (2009). https://doi.org/10.1038/nmat2317
Ni, M., Leung, M.K.H., Leung, D.Y.C., Sumathy, K.: A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 11(3), 401–425 (2007). https://doi.org/10.1016/j.rser.2005.01.009
Yu, H.J., Zhao, Y.F., Zhou, C., Shang, L., Peng, Y., Cao, Y., Wu, L.-Z., Tung, C.-H., Zhang, T.: Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2(10), 3344 (2014). https://doi.org/10.1039/c3ta14108j
Zhou, C., Zhao, Y., Bian, T., Shang, L., Yu, H., Wu, L.Z., Tung, C., Zhang, T.: Bubble template synthesis of Sn2Nb2O7 hollow spheres for enhanced visible-light-driven photocatalytic hydrogen production. Chem. Commun. (Camb) 49(84), 9872–9874 (2013). https://doi.org/10.1039/c3cc45683h
Tang, H.-L., Ren, Y., Wei, S.-H., Liu, G., Xu, X.-X.: Preparation of 3D ordered mesoporous anatase TiO2 and their photocatalytic activity. Rare Met. 38(5), 453–458 (2019). https://doi.org/10.1007/s12598-019-01211-8
Sajan, C.P., Wageh, S., Al-Ghamdi, A.A., Yu, J., Cao, S.: TiO2 nanosheets with exposed 001 facets for photocatalytic applications. Nano Res. 9(1), 3–27 (2015). https://doi.org/10.1007/s12274-015-0919-3
Sajan, C.P., Naik, A., Girish, H.N., Ravi, H.R., Singh, R.: Template-free processing of Ag-anchored ZnO polyscale sheets and their application in the photocatalytic degradation of organics present in pharmaceutical waste. Water Conserv. Sci. Eng. 2(2), 31–41 (2017). https://doi.org/10.1007/s41101-017-0022-6
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972)
Sajan, C.P., Naik, A., Girish, H.N.: Hydrothermal fabrication of WO3-modified TiO2 crystals and their efficiency in photocatalytic degradation of FCF. Int. J. Environ. Sci. Technol. 14(7), 1513–1524 (2017). https://doi.org/10.1007/s13762-016-1239-1
Wang, X., Li, X.-Y.: Concurrent photocatalytic hydrogen production and organic degradation by a composite catalyst film in a two-chamber photo-reactor. Water Sci. Technol. 67(12), 2845–2849 (2013). https://doi.org/10.2166/wst.2013.197
Dhakshinamoorthy, A., Navalon, S., Corma, A., Garcia, H.: Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 5(11), 9217–9233 (2012). https://doi.org/10.1039/c2ee21948d
Hu, J., Cao, Y., Wang, K., Jia, D.: Green solid-state synthesis and photocatalytic hydrogen production activity of anatase TiO2 nanoplates with super heat-stability. RSC Adv. 7, 11827–11833 (2017). https://doi.org/10.1039/C6RA27160J
Yang, D.-X., Qu, D., Miao, X., Jiang, W.-S., An, L., Wen, Y.-J., Wu, D.-D., Sun, Z.-C.: TiO2 sensitized by red-, green-, blue-emissive carbon dots for enhanced H2 production. Rare Met. 38(5), 404–412 (2019). https://doi.org/10.1007/s12598-019-01236-z
Wang, L., Jin, P.X., Shuhua, D., She, H., Huang, J., Wang, Q.Z.: In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci. Bull. 64(13), 926–933 (2019). https://doi.org/10.1016/j.scib.2019.05.012
Gupta, S.M., Tripathi, M.: A review of TiO2 nanoparticles. Chin. Sci. Bull. 56(16), 1639–1657 (2011). https://doi.org/10.1007/s11434-011-4476-1
Lan, Y., Lu, Y., Ren, Z.: Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2(5), 1031–1045 (2013). https://doi.org/10.1016/j.nanoen.2013.04.002
Schneider, J., Matsuoka, M., Takeuchi, M., Zhang, J., Horiuchi, Y., Anpo, M., Bahnemann, D.W.: Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 114(19), 9919–9986 (2014). https://doi.org/10.1021/cr5001892
Liu, Q., Hu, J., Liang, Y., Guan, Z.-C., Zhang, H., Hai-Peng, W., Duz, R.-G.: Preparation of MoO3/TiO2 composite films and their application in photoelectrochemical anticorrosion. J. Electrochem. Soc. 163(9), C539–C544 (2016). https://doi.org/10.1149/2.0481609jes
Wu, J.-J., Yu, C.-C.: Aligned TiO2 nanorods and nanowalls. J. Phys. Chem. B 108(11), 3378–3379 (2004)
Choi, H., Al-Abed, S., Dionysiou, D.D., Stathatos, E., Lianos, P.: TiO2-based advanced oxidation nanotechnologies for water purification and reuse. Sustain. Sci. Eng. 2, 229–254 (2010). https://doi.org/10.1016/s1871-2711(09)00208-6
Luo, L.J., Li, J., Dai, J., Xia, L., Barrow, C., Wang, H., Jegatheesan, J., Yang, M.: Bisphenol A removal on TiO2–MoS2-reduced graphene oxide composite by adsorption and photocatalysis. Process Saf. Environ. Prot. 112, 274–279 (2017). https://doi.org/10.1016/j.psep.2017.04.032
Ali, S.M.Y.M.M., Sandhya, K.Y.: Highly active TiO2–MoS2 composite for visible light photocatalytic applications. Mater. Sci. Forum 830831, 553–556 (2015). https://doi.org/10.4028/scientific.net/MSF.830-831.553
Liu, H., Lv, T., Zhu, C., Su, X., Zhu, Z.: Efficient synthesis of MoS2 nanoparticles modified TiO2 nanobelts with enhanced visible-light-driven photocatalytic activity. J. Mol. Catal. A: Chem. 396, 136–142 (2015). https://doi.org/10.1016/j.molcata.2014.10.002
Liu, J., Li, Y., Ke, J., Wang, Z., Xiao, H.: Synergically improving light harvesting and charge transportation of TiO2 nanobelts by deposition of MoS2 for enhanced photocatalytic removal of Cr(VI). Catalysts 7(1), 1–13 (2017). https://doi.org/10.3390/catal7010030
Hu, K.H., Hu, X.G., Xu, Y.F., Sun, J.D.: Synthesis of nano-MoS2/TiO2 composite and its catalytic degradation effect on methyl orange. J. Mater. Sci. 45(10), 2640–2648 (2010). https://doi.org/10.1007/s10853-010-4242-9
Sabarinathan, M., Harish, S., Archana, J., Navaneethan, M., Ikedab, H., Hayakawa, Y.: Highly efficient visible-light photocatalytic activity of MoS2–TiO2 mixtures hybrid photocatalyst and functional properties. RSC Adv. 7, 24754–24763 (2017). https://doi.org/10.1039/C7RA03633G
Aray, Y., Rodríguez, J.: Atoms in molecules theory for exploring the nature of the MoS2 catalyst edges sites. J. Mol. Catal. A: Chem. 265(1–2), 32–41 (2007). https://doi.org/10.1016/j.molcata.2006.09.034
Ren, X., Qi, X., Shen, Y., Xiao, S., Xu, G., Zhang, Z., Huang, Z., Zhong, J.: 2D co-catalytic MoS2 nanosheets embedded with 1D TiO2 nanoparticles for enhancing photocatalytic activity. J. Phys. D Appl. Phys. 49(31), 315304 (2016). https://doi.org/10.1088/0022-3727/49/31/315304
Liu, C., Wang, L., Tang, Y., Luo, S., Liu, Y., Zhang, S., Zeng, Y., Xu, Y.: Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B 164, 1–9 (2015). https://doi.org/10.1016/j.apcatb.2014.08.046
He, H., Lin, J., Fu, W., Wang, X., Wang, H., Zeng, Q., Gu, Q., Li, Y., Yan, C., Tay, B.K., Xue, C., Hu, X., Pantelides, S.T., Zhou, W., Liu, Z.: MoS2/TiO2 edge-on heterostructure for efficient photocatalytic hydrogen evolution. Adv. Energy Mater. 6(14), 1600464 (2016). https://doi.org/10.1002/aenm.201600464
Li, W., Liang, R., Hu, A., Huang, Z., Zhou, Y.N.: Generation of oxygen vacancies in visible light-activated one-dimensional iodine TiO2 photocatalysts. RSC Adv. 4, 36959–36966 (2014). https://doi.org/10.1039/C4RA04768K
Yang, L., Cui, X., Zhang, J., Wang, K., Shen, M., Zeng, S., Dayeh, S.A., Feng, L., Xiang, B.: Lattice strain effects on the optical properties of MoS2 nanosheets. Sci. Rep. 4, 1–7 (2014). https://doi.org/10.1038/srep05649
Zeng, H.C.: Ostwald ripening: a synthetic approach for hollow nanomaterials. Curr. Nanosci. 3, 177–181 (2007)
Jiang, Z., Liu, Y., Jing, T., Huang, B., Wang, Z., Zhang, X., Qin, X., Dai, Y.: Enhancing visible light photocatalytic activity of TiO2 using a colorless molecule (2-methoxyethanol) due to hydrogen bond effect. Appl. Catal. B 200, 230–236 (2017). https://doi.org/10.1016/j.apcatb.2016.07.009
Liu, J., Han, L., An, N., Xing, L., Ma, H., Cheng, L., Yang, J., Zhang, Q.: Enhanced visible-light photocatalytic activity of carbonate-doped anatase TiO2 based on the electron-withdrawing bidentate carboxylate linkage. Appl. Catal. B 202, 642–652 (2017). https://doi.org/10.1016/j.apcatb.2016.09.057
Ivanova, E., Mihaylov, M., Thibault-Starzyk, F., Daturi, M., Hadjiivanov, K.: FTIR spectroscopy study of CO and NO adsorption and co-adsorption on Pt/TiO2. J. Mol. Catal. A: Chem. 274(1–2), 179–184 (2007). https://doi.org/10.1016/j.molcata.2007.05.006
Stojanović, D.B., Brajović, L., Orlović, A., Dramlić, D., Radmilović, V., Uskoković, P.S., Aleksić, R.: Transparent PMMA/silica nanocomposites containing silica nanoparticles coating under supercritical conditions. Prog. Org. Coat. 76(4), 626–631 (2013). https://doi.org/10.1016/j.porgcoat.2012.12.002
Akhavan, O., Abdolahad, M., Esfandiar, A., Mohatashamifar, M.: Photodegradation of graphene oxide sheets by TiO2 nanoparticles after a photocatalytic reduction. J. Phys. Chem. C 114, 12955–12959 (2010)
Jaramillo, T.F., Jørgensen, K.P., Bonde, J., Nielsen, J.H., Horch, S., Chorkendorff, I.: Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007)
Meissner, D., Memming, R.: Photoelectrochemistry of cadmium sulfide. 1. Reanalysis of photocorrosion and flat-band potential. Am. Chem. Soc. 92, 3476–3483 (1987)
Thurston, T.R., Wilcoxon, J.P.: Photooxidation of Organic chemicals catalyzed by nanoscale MoS2. J. Phys. Chem. B 103, 11–17 (1999)
Yu, J., Zhang, J.: A simple template-free approach to TiO2 hollow spheres with enhanced photocatalytic activity. Dalton Trans. 39(25), 5860–5867 (2010). https://doi.org/10.1039/c0dt00053a
Acknowledgements
The authors wish to thank all who assisted in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chimmikuttanda, S.P., Akple, M.S., Naik, A. et al. Fabrication of MoS2-deposited TiO2 hollow microspheres and their enhanced photocatalytic application in the generation of hydrogen. Mater Renew Sustain Energy 9, 22 (2020). https://doi.org/10.1007/s40243-020-00182-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-020-00182-6